- Department of Neuroradiology, The Royal London Hospital, London, UK
- Neuroradiological Clinic, Neurocenter, Leipzig, Germany
- Department for Neuroradiology, University Hospital Leipzig, Leipzig, Germany
- Neurological Clinic, Neurocenter, Leipzig, Germany
- Neurosurgical Clinic, Neurocenter, Klinikum Stuttgart, Leipzig, Germany
- Medical Faculty, University Duisburg-Essen, Duisburg, Germany
Correspondence Address:
Pervinder Bhogal
Neuroradiological Clinic, Neurocenter, Leipzig, Germany
DOI:10.4103/sni.sni_243_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Pervinder Bhogal, Elina Henkes, Stefan Schob, Muhammad AlMatter, Victoria Hellstern, Hansjörg Bäzner, Oliver Ganslandt, Hans Henkes, Marta Aguilar Pérez. The use of flow diverters to treat small (≤5 mm) ruptured, saccular aneurysms. 30-Oct-2018;9:216
How to cite this URL: Pervinder Bhogal, Elina Henkes, Stefan Schob, Muhammad AlMatter, Victoria Hellstern, Hansjörg Bäzner, Oliver Ganslandt, Hans Henkes, Marta Aguilar Pérez. The use of flow diverters to treat small (≤5 mm) ruptured, saccular aneurysms. 30-Oct-2018;9:216. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9051
Abstract
Background:There is limited published literature on the use of flow diverting stents (FDS) to treat ruptured intracranial aneurysms in the acute stage. We present our experience of using FDS to treat small (≤5 mm) ruptured aneurysms.
Methods:We retrospectively identified all patients with ≤5 mm ruptured aneurysms treated exclusively with FDS between February 2009 and February 2016. We recorded demographic data, the Hunt and Hess score, aneurysm location and size, therapeutic intervention, immediate angiographic and clinical result, and clinical and radiological follow-up information.
Results:We identified seven patients (four females) with average age 59.8 ± 10 years (range 48–75). The average aneurysm fundus size was 2.7 ± 0.76 mm (range 1–4 mm). The average time from ictus to treatment was 6.3 days (range 1–14 days) and there were no cases of repeat rupture prior to treatment or intraoperative rupture. Angiographic follow-up was available in five patients. At initial follow-up, aneurysms (100%) were completely occluded raymond roy classification 1 (RRC 1). None of the aneurysms re-ruptured following treatment. Clinically, six patients were discharged with good functional outcome modified Rankin Score (mRS ≤2). There were no mortalities.
Conclusion:The use of FDS to treat small, ruptured, saccular aneurysms is feasible; however, the use of FDS should not be considered first-line treatment. Further studies are required to determine the safety and efficacy of the use of FDS in the acute situation.
Keywords: Flow diverter, ruptured aneurysm, subarachnoid haemorrhage
INTRODUCTION
The introduction of flow diverting stents (FDS) represented a paradigm shift in the way intracranial aneurysms were treated and for the first time a treatment option that allowed reconstruction of the diseased parent artery became available. Although the exact mechanism of action by which flow diverters act is debated, there is a general consensus that initially these devices alter the intra-aneurysmal hemodynamics to promote thrombosis with subsequent formation of neo-intima over the braided stent wires and complete exclusion of the aneurysm from the circulation.[
In this study, we present our data on the treatment of acutely ruptured, small (≤5 mm), saccular aneurysms with FDS.
METHODS
Patient population
We searched our prospectively maintained database, for patients treated in our institution between February 2009 and February 2016, with ruptured, saccular aneurysms ≤5 mm in maximal size treated with FDS in the acute and early subacute period (≤14 days). Patients treated prior to the acute rupture with clipping, for example, ruptured remnant, were also included. Exclusion criteria included fusiform, blister, and dissecting aneurysms. We excluded aneurysms that were coiled acutely and then treated with flow diversion either during the same hospital admission or at a later date.
For each patient, we recorded demographic data, clinical presentation, aneurysm location, therapeutic intervention, immediate angiographic and clinical result, and clinical and radiological follow-up information.
Endovascular treatment
All treatments were performed under general anesthesia. A single type of flow diverter, the p64 (Phenox, Bochum, Germany), was used in all cases and this was based upon the higher mesh density compared to the Pipeline Embolization Device (PED) (Medtronic, Dublin, Ireland) which is the only other flow diverter available in our department.
Dependent upon the clinical state of the patient, premedication was given either on table or orally at least 3 h prior to the operation for patients able to take oral medications. For patients able to take oral medications, loading doses of aspirin (500 mg) and ticagrelor (180 mg) were given on the morning of the surgery. The effectiveness of the antiplatelet medication was tested using both the VerifyNow (Accumetrics) and Multiplate (Roche) analyzers to ensure adequate anti-aggregation prior to the operation and at least 3 h post-medication. For patients unable to take oral medication prior to the operation, an intravenous bolus dose of weight-adjusted eptifibatide was given on table. Subsequently, loading doses of ticagrelor via NG tube (180 mg) and IV aspirin (500 mg) were given at the end of the procedure. In both situations, the effectiveness of the antiplatelet medication was tested 24-h post-procedure using the VerifyNow and Multiplate analyzers.
The post-procedural antiplatelet regimen consisted of ticagrelor (90 mg twice daily) continued for 12 months following treatment and aspirin (100 mg once daily) continued for life.
The preoperative brain imaging was carefully assessed to determine if an external ventricular drain (EVD) was required or may be required. Signs of obstructive hydrocephalus, intraventricular blood, or evidence of raised intracranial pressure were used as markers to guide the insertion of an EVD. The EVD was inserted prior to the initiation of antiplatelet medication. Following insertion of the EVD, repeat imaging was performed to assess both the positioning of the EVD and to exclude hemorrhage along the EVD tract. Antiplatelet medication was commenced only after hemorrhage following drain insertion had been excluded.
All procedures were performed via the right common femoral route using a 6-Fr access system as standard. All procedures were performed under heparin anticoagulation with a 5000-IU bolus dose at the start of the procedure and subsequent 1000-IU bolus doses every hour to maintain the activated clotting time between 2 and 2.5 times the baseline.
Procedural assessment and follow-up
Patency and flow characteristics within the aneurysm and parent artery were assessed angiographically immediately after placement of the FDS and during follow-up. Follow-up axial imaging, either CT or MRI, were performed prior to the discharge of the patient. A CT angiogram was routinely performed if the patient developed signs or symptoms of delayed cerebral vasospasm.
Procedural follow-up was performed initially at 3–6 months, again at 9–12 months, and then once per year. Standard angiographic projections were used to assess the patency of the vessels and the aneurysms in addition to angiographic projections that repeated those used during the treatment. Aneurysm occlusion was graded as either completely excluded, minor remnant, major remnant, or unchanged (patent) and additionally using the 3-point Raymond–Roy classification.[
RESULTS
Population
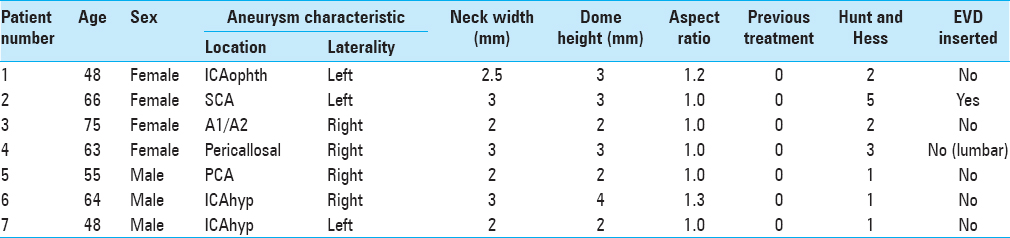
In total, we identified 320 patients with ruptured aneurysms ≤5 mm in size. We identified seven patients (four females) that met our inclusion and exclusion criteria. The average age of the patients was 59.8 ± 10 years (range 48–75). The average aneurysm fundus size was 2.7 ± 0.76 mm (range 1–4 mm). The average neck width was 2.5 ± 0.5 mm (range 1–5 mm) with average aspect ratio 1.1. The majority of aneurysms were located in the anterior circulation (n = 5) with three aneurysms located in the clinoidal or supraclinoidal segment of the internal carotid artery (ICA), one aneurysm located at the A1/A2 junction, and one aneurysm on the pericallosal artery. In the posterior circulation, one aneurysm was located on the superior cerebellar artery and one aneurysm on the posterior cerebral artery. None of the aneurysms was previously treated.
In terms of clinical presentation, three patients presented with Hunt and Hess grade 1, two patients with Hunt and Hess grade 2, one patient with Hunt and Hess grade 3, and one patient with Hunt and Hess grade 5 subarachnoid hemorrhage. One patient had an EVD inserted and in one patient a lumbar drain was inserted. There were no cases of hemorrhage secondary to drain insertion or following initiation of antiplatelet medication. The results are summarized in
Feasibility
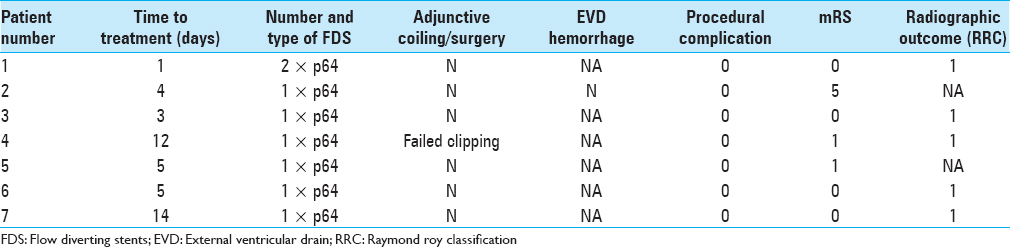
Delivery of the flow diverter was feasible in all cases. The p64 was used in all cases. In a single patient, two p64 flow diverters were used. In the remaining six patients, a single FDS was deployed. The average time from ictus to treatment was 6.3 days (range 1–14 days) and there were no cases of repeat rupture prior to treatment. Treatment was significantly delayed in two patients (patient 4 and 7). In patient 4, this was due to unsuccessful surgical attempt at clipping and then the development of vasospasm. In the patient 7, the patient presented in a delayed fashion and initially remained undiagnosed in a nonspecialist hospital.
The use of flow diversion was taken after multidisciplinary team discussion in six cases and based principally around the unfavorable morphology and anatomical configuration of the aneurysm. In all cases, the aneurysms had unfavorable aspect ratios, ≤1.3 in all cases, which would have likely necessitated the use of stent-assisted coiling. Furthermore, given the small size of the aneurysms (2.7 ± 0.75 mm), we felt that catheterization of the aneurysm, without the protection of a balloon, would pose a risk of intraoperative rupture. In one case, patient 4, the patient initially went to surgery. At the time of surgery, the rupture point of the aneurysm was found to be at neck of the aneurysm and therefore clipping of the aneurysm would have involved occlusion of the pericallosal artery. Similarly, because of the rupture point, endovascular coiling was also felt to be high risk.
There were no cases of intraoperative aneurysm rupture and there were no intraoperative complications.
Angiographic and clinical follow-up
Angiographic follow-up was available in five patients. At initial follow-up performed on average at 3.4 months after the procedure, five aneurysms (100%) were completely occluded (RRC 1). In two patients, there is no follow-up angiographic imaging available [Figures
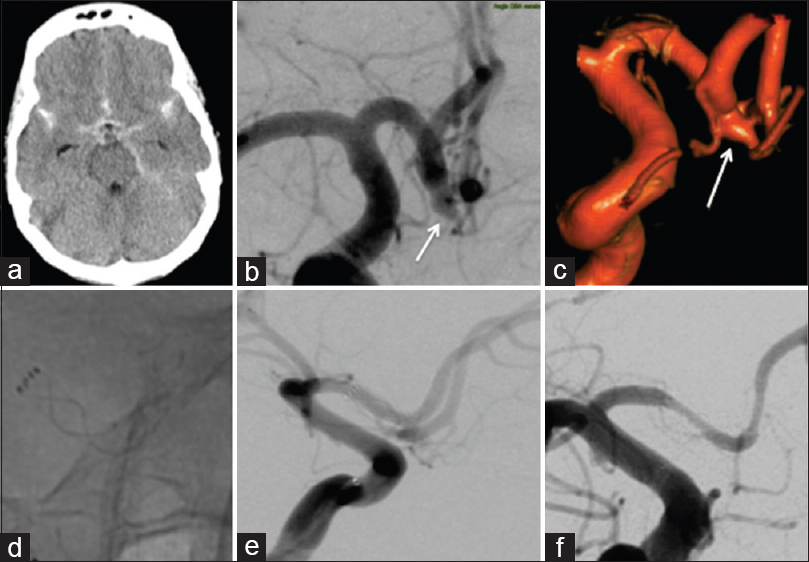
Figure 1
Patient 4 presented with diffuse subarachnoid hemorrhage (a) and a solitary aneurysm of the A1/2 junction [(b and c) white arrows). After an attempted clipping, the patient was referred for endovascular treatment with a single p64 flow diverter (d and e). There were no intraoperative complications and there was no evidence of recurrent hemorrhage. Follow-up angiography at 4 months revealed complete exclusion of the aneurysm and asymptomatic, mild/moderate in stent stenosis (f)
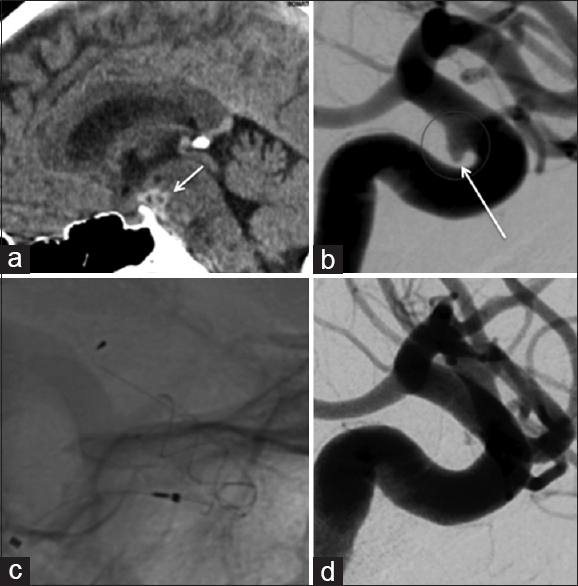
Figure 2
Patient 6 presented with a localized subarachnoid hemorrhage (a, short white arrow) and a solitary aneurysm of the ICA (b, long white arrow). The wide neck would have necessitated stent-assisted coiling, and therefore, flow diversion was thought to represent a safer treatment option (c). Follow-up angiography at 3 months showed virtually complete exclusion of the aneurysm from the circulation (d). There was no evidence of repeat hemorrhage and no clinical or radiological complications following the implantation of the p64 flow diverter
None of the aneurysms re-ruptured following treatment. Clinically, six patients were discharged with good functional outcome (mRS ≤2), and the remaining patient was discharged with mRS 5, which was her baseline neurological status. There were no mortalities. The results are summarized in
DISCUSSION
Stent-assisted coiling is a widely used and accepted treatment option for unruptured intracranial aneurysms; however, the use of stents in the acute situation is generally avoided unless absolutely necessary. The use of stents, either alone or in conjunction with endovascular coiling, in the acute situation is believed to expose patients to an elevated risk of bleeding-related complications if interventions such as the insertion of an EVD are required.[
Several studies have documented the use of FDS in the acute situation,[
When considering the benefits and risks of FDS as a treatment option for small, ruptured aneurysms, it is important to compare with the risks of standard endovascular coiling. These risks relate to the difficulty in obtaining a stable microcatheter position as well as to the perceived increased risk of perforation related to the small, confined space. However, aneurysms smaller than 3 mm are routinely coiled and advances in imaging, increasing operator experience, and the widespread use of adjunctive devices have all assisted in making coiling of these tiny aneurysms feasible. Recently, Brinjikji et al.[
The introduction of surface-modified FDS with the requirement for only a single antiplatelet medication may prove particularly useful in the acute situation. We are aware of only a single publication documenting the use of these new devices in an acutely ruptured fusiform aneurysm.[
The retrospective design and small numbers limit our study. It is a single-center study and all the aneurysms were treated with a single type of FDS; extrapolation of these results to other types of FDS may not be feasible. Although we focused our analysis on small aneurysms, the technique could be used for larger aneurysms; however, again the applicability of our results is difficult to determine. Furthermore, as all the aneurysms are saccular, we are unsure of the applicability of the technique to fusiform aneurysms or to blister aneurysms.
CONCLUSION
The use of FDS to treat small, ruptured, saccular aneurysms is feasible, and in our small series, we achieved reasonable radiographic and clinical outcomes with no cases of re-rupture. Although the use of FDS should not be considered first-line treatment, it represents a potential alternative treatment option when standard endovascular coiling or neurosurgery may not be feasible.
Financial support and sponsorship
Nil.
Conflicts of interest
PB and MAP serve as proctors and consultant for Phenox. HH is share holder and co-founder of Phenox.
References
1. Alfke K, Straube T, Dörner L, Mehdorn HM, Jansen O. Treatment of intracranial broad-neck aneurysms with a new self-expanding stent and coil embolization. AJNR Am J Neuroradiol. 2004. 25: 584-91
2. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: Five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017. 80: 40-8
3. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology. 2013. 267: 858-68
4. Bhogal P, Aguilar Pérez M, Sauder G, Bäzner H, Ganslandt O, Henkes H. Management of paraophthalmic aneurysms : Review of endovascular treatment strategies. Ophthalmologe. 2018. 115: 114-22
5. Bhogal P, Martinez Moreno R, Ganslandt O, Bäzner H, Henkes H, Perez MA. Use of flow diverters in the treatment of unruptured saccular aneurysms of the anterior cerebral artery. J Neurointerv Surg. 2017. 9: 283-9
6. Bhogal P, Pérez MA, Ganslandt O, Bäzner H, Henkes H, Fischer S. Treatment of posterior circulation non-saccular aneurysms with flow diverters: A single-center experience and review of 56 patients. J Neurointerv Surg. 2017. 9: 471-81
7. Bhogal P, AlMatter M, Bäzner H, Ganslandt O, Henkes H, Aguilar Pérez M. Flow diversion for the treatment of MCA bifurcation aneurysms-A single centre experience. Front Neurol. 2017. 8: 20-
8. Bhogal P, Ganslandt O, Bäzner H, Henkes H, Pérez MA. The fate of side branches covered by flow diverters-results from 140 patients. World Neurosurg. 2017. 103: 789-98
9. Bhogal P, Martinez R, Gansladt O, Bäzner H, Henkes H, Aguilar M. Management of unruptured saccular aneurysms of the M1 Segment with flow diversion: A single centre experience. Clin Neuroradiol. 2018. 28: 209-16
10. Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: Strategies in stent deployment and midterm follow-up. Neurosurgery. 2007. 61: 460-8
11. Bodily KD, Cloft HJ, Lanzino G, Fiorella DJ, White PM, Kallmes DF. Stent-assisted coiling in acutely ruptured intracranial aneurysms: A qualitative, systematic review of the literature. AJNR Am J Neuroradiol. 2011. 32: 1232-6
12. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J. 2015. 28: 365-75
13. Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Boccardi E, Cekirge S. Risk factors for ischemic complications following pipeline embolization device treatment of intracranial aneurysms: Results from the intrePED study. AJNR Am J Neuroradiol. 2016. 37: 1673-8
14. Brinjikji W, Lanzino G, Cloft HJ, Rabinstein A, Kallmes DF. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: Report of a consecutive series and a meta-analysis. Stroke. 2010. 41: 116-21
15. Cebral JR, Mut F, Raschi M, Hodis S, Ding YH, Erickson BJ. Analysis of hemodynamics and aneurysm occlusion after flow-diverting treatment in rabbit models. AJNR Am J Neuroradiol. 2014. 35: 1567-73
16. Chalouhi N, Starke RM, Yang S, Bovenzi CD, Tjoumakaris S, Hasan D. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke. 2014. 45: 54-8
17. Chalouhi N, Zanaty M, Whiting A, Tjoumakaris S, Hasan D, Ajiboye N. Treatment of ruptured intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2015. 76: 165-72
18. Chan RS, Mak CH, Wong AK, Chan KY, Leung KM. Use of the pipeline embolization device to treat recently ruptured dissecting cerebral aneurysms. Interv Neuroradiol. 2014. 20: 436-41
19. Cruz JP, O’Kelly C, Kelly M, Wong JH, Alshaya W, Martin A. Pipeline embolization device in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2013. 34: 271-6
20. De Vries J, Boogaarts J, Van Norden A, Wakhloo AK. New generation of flow diverter (surpass) for unruptured intracranial aneurysms: A prospective single-center study in 37 patients. Stroke. 2013. 44: 1567-77
21. Fiorella D, Lylyk P, Szikora I, Kelly ME, Albuquerque FC, McDougall CG. Curative cerebrovascular reconstruction with the pipeline embolization device: The emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. 2009. 1: 56-65
22. Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct: Case report. Neurosurgery. 2010. 67: onsE313-4
23. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012. 54: 369-82
24. Hagen MW, Girdhar G, Wainwright J, Hinds MT. Thrombogenicity of flow diverters in an ex vivo shunt model: Effect of phosphorylcholine surface modification. J Neurointerv Surg. 2017. 9: 1006-11
25. Hanel RA, Aguilar-Salinas P, Brasiliense LB, Sauvageau E. First US experience with pipeline flex with shield technology using aspirin as antiplatelet monotherapy. BMJ Case Rep 2017. 2017. p.
26. Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014. 270: 394-9
27. Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P. Safety and efficacy of the pipeline embolization device for treatment of intracranial aneurysms: A pooled analysis of 3 large studies. J Neurosurg. 2017. 127: 775-80
28. Katsaridis V, Papagiannaki C, Violaris C. Embolization of acutely ruptured and unruptured wide-necked cerebral aneurysms using the neuroform2 stent without pretreatment with antiplatelets: A single center experience. AJNR Am J Neuroradiol. 2006. 27: 1123-8
29. Kocer N, Islak C, Kizilkilic O, Kocak B, Saglam M, Tureci E. Flow re-direction endoluminal device in treatment of cerebral aneurysms: Initial experience with short-term follow-up results. J Neurosurg. 2014. 120: 1158-71
30. Kung DK, Policeni BA, Capuano AW, Rossen JD, Jabbour PM, Torner JC. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J Neurosurg. 2011. 114: 1021-7
31. Lin N, Brouillard AM, Keigher KM, Lopes DK, Binning MJ, Liebman KM. Utilization of pipeline embolization device for treatment of ruptured intracranial aneurysms: US multicenter experience. J Neurointerv Surg. 2015. 7: 808-15
32. Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F. Silk flow-diverter stent for the treatment of intracranial aneurysms: A series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol. 2015. 36: 542-6
33. Madaelil TP, Wallace AN, Chatterjee AN, Zipfel GJ, Dacey RG, Cross DT. Endovascular parent vessel sacrifice in ruptured dissecting vertebral and posterior inferior cerebellar artery aneurysms: Clinical outcomes and review of the literature. J Neurointerv Surg. 2016. 8: 796-801
34. Maimon S, Gonen L, Nossek E, Strauss I, Levite R, Ram Z. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien). 2012. 154: 979-87
35. Marosfoi M, Clarencon F, Langan ET, King RM, Brooks OW, Tamura T. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. 2018. 10: 406-11
36. McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2012. 33: 487-93
37. Mocco J, Snyder KV, Albuquerque FC, Bendok BR, Alan S B, Carpenter JS. Treatment of intracranial aneurysms with the enterprise stent: A multicenter registry. J Neurosurg. 2009. 110: 35-9
38. Möhlenbruch MA, Herweh C, Jestaedt L, Stampfl S, Schönenberger S, Ringleb PA. The FRED flow-diverter stent for intracranial aneurysms: Clinical study to assess safety and efficacy. AJNR Am J Neuroradiol. 2015. 36: 1155-61
39. Mpotsaris A, Skalej M, Beuing O, Eckert B, Behme D, Weber W. Long-term occlusion results with SILK flow diversion in 28 aneurysms: Do recanalizations occur during follow-up?. Interv Neuroradiol. 2015. 21: 300-10
40. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011. 32: 34-40
41. Nerva JD, Morton RP, Levitt MR, Osbun JW, Ferreira MJ, Ghodke BV. Pipeline embolization device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg. 2015. 7: 210-6
42. O’Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2013. 34: 381-7
43. Rajah G, Narayanan S, Rangel-Castilla L. Update on flow diverters for the endovascular management of cerebral aneurysms. Neurosurg Focus. 2017. 42: E2-
44. Rammos S, Klopfenstein J, Augspurger L, Wang H, Wagenbach A, Poston J. Conversion of external ventricular drains to ventriculoperitoneal shunts after aneurysmal subarachnoid hemorrhage: Effects of site and protein/red blood cell counts on shunt infection and malfunction. J Neurosurg. 2008. 109: 1001-4
45. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001. 32: 1998-2004
46. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: A single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012. 33: 1436-46
47. Sani S, Jobe KW, Lopes DK. Treatment of wide-necked cerebral aneurysms with the neuroform2 treo stent. A prospective 6-month study. Neurosurg Focus. 2005. 18: E4-
48. dos Santos Souza MP, Agid R, Willinsky RA, Cusimano M, Montanera W, Wallace MC. Microstent-assisted coiling for wide-necked intracranial aneurysms. Can J Neurol Sci. 2005. 32: 71-81
49. Strauss I, Maimon S. Silk flow diverter in the treatment of complex intracranial aneurysms: A single-center experience with 60 patients. Acta Neurochir (Wien). 2016. 158: 247-54
50. Tumialán LM, Zhang YJ, Cawley CM, Dion JE, Tong FC, Barrow DL. Intracranial hemorrhage associated with stent-assisted coil embolization of cerebral aneurysms: A cautionary report. J Neurosurg. 2008. 108: 1122-9
51. Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A. Surpass flow diverter in the treatment of intracranial aneurysms: A prospective multicenter study. AJNR Am J Neuroradiol. 2015. 36: 98-107
52. Yoon JW, Siddiqui AH, Dumont TM, Levy EI, Hopkins LN, Lanzino G. Feasibility and safety of pipeline embolization device in patients with ruptured carotid blister aneurysms. Neurosurgery. 2014. 75: 419-29
53. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM. Intracranial aneurysms: Midterm outcome of pipeline embolization device – A prospective study in 143 patients with 178 aneurysms. Radiology. 2012. 265: 893-901
54. Zhou Y, Yang PF, Fang YB, Xu Y, Hong B, Zhao WY. Anovel flow-diverting device (Tubridge) for the treatment of 28 large or giant intracranial aneurysms: A single-center experience. AJNR Am J Neuroradiol. 2014. 35: 2326-33
55. Zhu Y, Pan J, Shen J, Liu C, Fan Z, Shen Y. Clinical and radiological outcomes after treatment of unruptured paraophthalmic internal carotid artery aneurysms: A Comparative and pooled analysis of single-center experiences. World Neurosurg. 2015. 84: 1726-38