- Department of Neurosurgery, University of the Ryukyus Faculty of Medicine Hospital, Okinawa, Japan
Correspondence Address:
Shogo Ishiuchi
Department of Neurosurgery, University of the Ryukyus Faculty of Medicine Hospital, Okinawa, Japan
DOI:10.4103/sni.sni_19_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Takahiro Shinya, Hideki Nagamine, Ken-ichi Sugawara, Shogo Ishiuchi. The usefulness of indocyanine green during surgery for hypervascular posterior fossa tumors. 26-Apr-2018;9:90
How to cite this URL: Takahiro Shinya, Hideki Nagamine, Ken-ichi Sugawara, Shogo Ishiuchi. The usefulness of indocyanine green during surgery for hypervascular posterior fossa tumors. 26-Apr-2018;9:90. Available from: http://surgicalneurologyint.com/surgicalint-articles/the-usefulness-of-indocyanine-green-during-surgery-for-hypervascular-posterior-fossa-tumors/
Abstract
Background:Cerebral hemangioblastomas are benign tumors with abundant blood flow that occur mainly in the posterior fossa. Tumor removal en bloc is important in surgical treatment because of the risk of bleeding; however, it is actually rather difficult in practice. Therefore, we propose a surgical strategy for visualizing hypervascular tumors of the posterior fossa utilizing indocyanine green (ICG).
Case Description:Case 1 involved a 48-year-old male with a history of von Hippel–Lindau (VHL) disease. Magnetic resonance imaging (MRI) revealed a solid tumor measuring 3.0 cm in diameter in the right cerebellopontine angle. We performed surgery because the tumor was pressing against the brainstem. Surgery was performed via the posterior subtemporal transtentorial approach in order to visualize the feeding artery and draining vein intraoperatively. The vessels were confirmed by ICG and the tumor was removed en bloc. Case 2 involved a 30-year-old woman. Signs of increased intracranial pressure were noted, and an MRI revealed a solid tumor 3.5 cm in diameter in the left cerebellar hemisphere. Surgery was performed via the midline suboccipital approach. Similarly, we confirmed the vessels using ICG and the tumor was removed en bloc.
Conclusions:For hypervascular tumors of the posterior fossa, preoperative image assessment is important. Furthermore, the use of ICG during surgery is advantageous for surgical strategies where the feeding arteries and draining veins exist superficially in the operative field and are therefore easier to remove en bloc.
Keywords: Hemangioblastoma, hypervascular, indocyanine green, surgical videoangiography
INTRODUCTION
In high vascular tumors of the posterior fossa, surgical resection can be extremely challenging. Hemangioblastomas are common hypervascular tumors that occur in the posterior fossa. The prevalence of hemangioblastoma has been estimated to be approximately 1.5–2.5% of all intracranial tumors and 7–8% of posterior fossa tumors.[
CASE HISTORY
Case 1
A 48-year-old male patient with a family history of VHL disease consulted an ophthalmologist because he had experienced blurred vision for 2 months prior to consultation. A diagnosis of bilateral retinal angioblastoma was made. Subsequently, magnetic resonance imaging (MRI) brain was performed to assess the patient for VHL. MRI showed a predominantly solid tumor with a maximum diameter of 3.0 cm accompanying an internal cyst close to the right cerebellopontine angle. In addition, edema extending to the brainstem was noted. The solid portion of the lesion was uniformly contrast-enhancing according to an MRI. Subsequent cerebral angiography identified feeding arteries from the superior cerebellar artery (SCA) and draining veins that converged onto the tentorial sinus [
Figure 1
The presurgical magnetic resonance (MR) image depicting an enhanced solid haemangioblastoma in the cerebellopontine angle (a). Postsurgical MR image depicting the resection of the tumour (b). Right vertebral artery angiogram exhibiting a hypervascular tumour near the superior cerebellar artery (c and d)
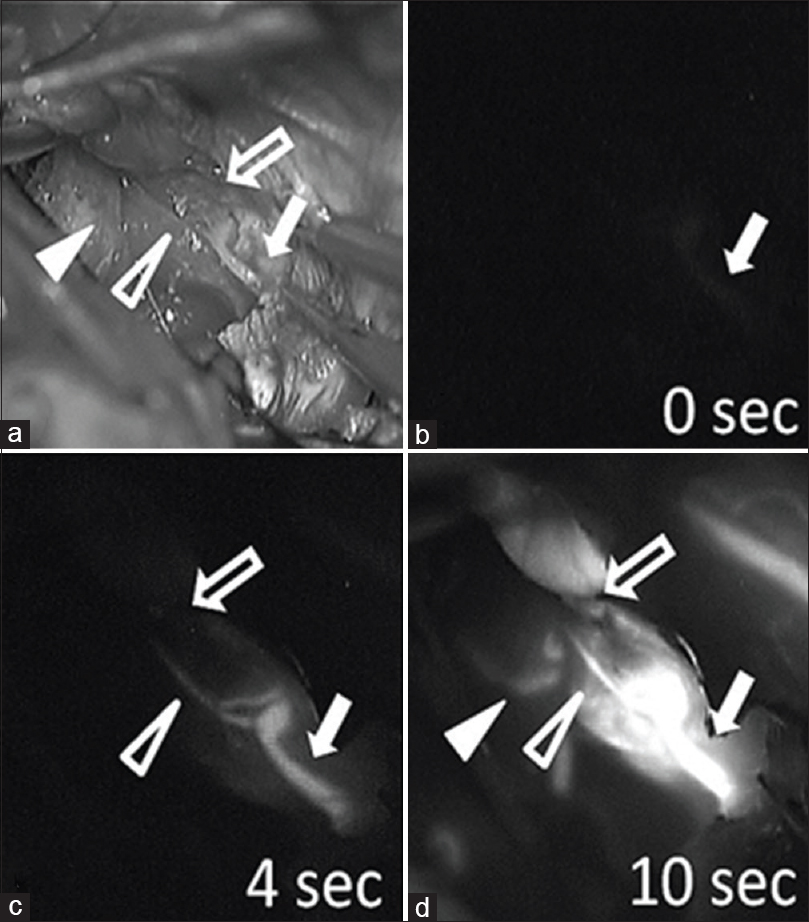
Surgery was performed via the posterior subtemporal transtentorial approach with the patient in the left supine lateral position. We intravenously injected mannitol and inserted a ventricular drainage tube into the supramarginal gyrus due to brain relaxation. After opening the dura mater, a surgical corridor was created by dissecting the bridging vein, and the tumor was confirmed by the retraction of the temporal lobe. The tumor was detached from the quadrigeminal bodies, followed by ICG fluorescence vascular angiography. First, the feeding arteries from the SCA were visualized, then the draining veins as they were converging with the tentorial sinus, and finally the transit arteries were carefully identified [
Figure 2
Surgical view (a) and indocyanine green (ICG) videography (b-d) during an operation. The main feeding arteries (arrow) are first filled with ICG (b), followed by transit feeders (open arrowhead), and then by simultaneous filling of drainers (open arrow) approximately 4 seconds after the main feeding arteries are visualized (c). Finally, non-feeding arteries (arrowhead) are filled with ICG approximately 10 seconds after the main feeding arteries (d)
Case 2
A 30-year-old woman patient visited our outpatient clinic after experiencing headache and blurred vision for 1 month. Her neurological examination was within the normal limits, except that bilateral congested papillae were identified. Her family history was unavailable because she was adopted. MRI revealed a solid tumor with a maximum diameter of 3.5 cm accompanying an internal cyst in the left cerebellar hemisphere. In addition, extensive cerebral edema and ventricular dilation were noted. On presurgical brain MRI, the solid region showed uniform contrast enhancement. On cerebral angiography, feeding arteries were noted to arise from the left anterior inferior cerebellar artery (AICA) and left posterior inferior cerebellar artery (PICA), and both the draining veins to the petrosal vein and the inferior vermian vein were identified [
Figure 3
The presurgical magnetic resonance (MR) image demonstrating the enhanced solid haemangioblastoma in the cerebellum (a). The postsurgical MR image demonstrating the resection of the tumour (b). A Three-dimensional computed tomography angiogram during the late phase, exhibiting a draining vein (open arrowhead) flowing into the petrosal vein on the ventral side of the tumour (c). Left vertebral artery angiogram exhibiting a hypervascular tumour near the anterior inferior cerebellar artery and the posterior inferior cerebellar artery (d and e)
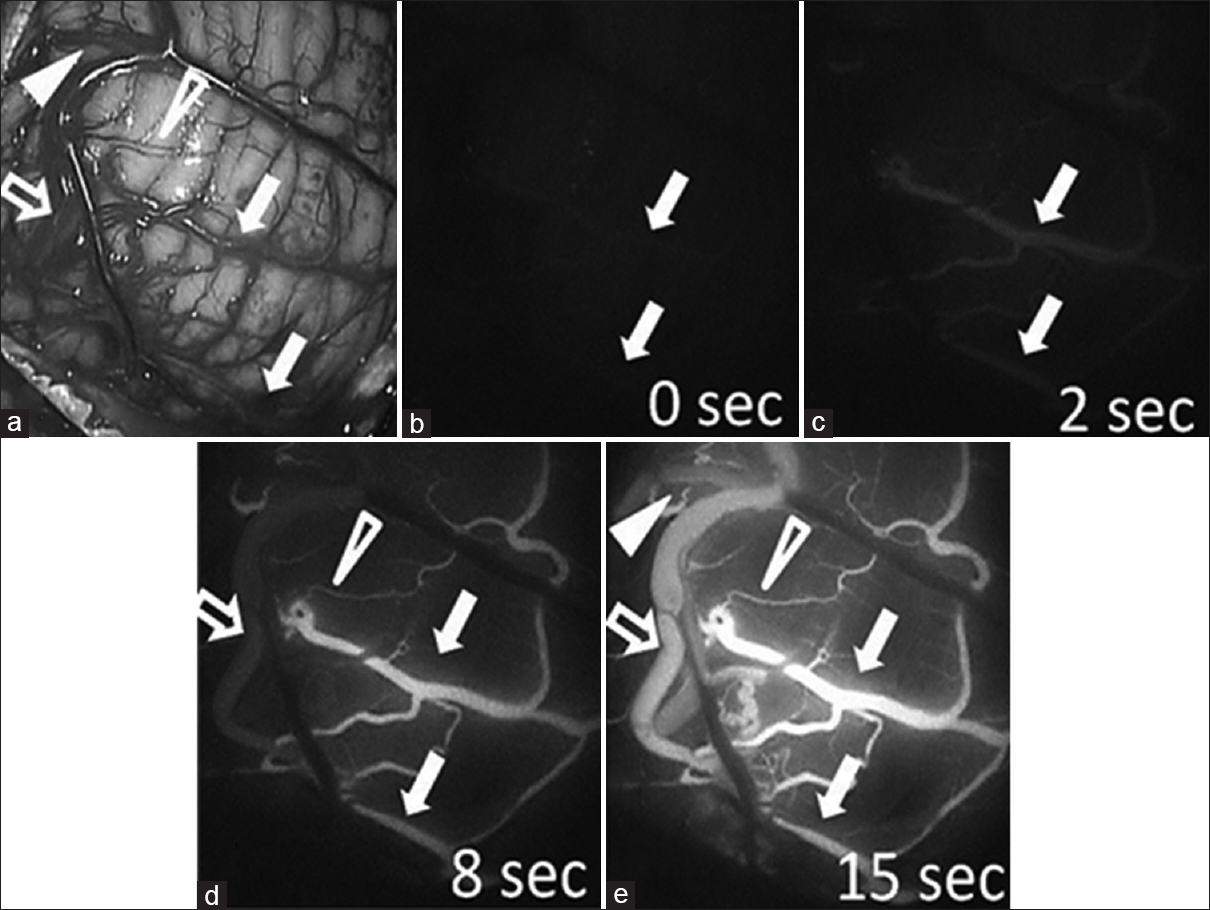
We performed 200 ml of 10% glycerol (twice a day) with dexamethasone (8 mg/day) for 6 days before the operation. Because the symptoms improved, we did not perform external ventricular drainage prior to the operation. Surgery was performed via the midline suboccipital approach with the patient in the prone position. The dura mater was dissected and ICG fluorescence angiography was performed. We observed the feeding artery coming off the PICA arose from the cerebellar hemisphere and drained via the horizontal fissure, additionally feeding vessels from the AICA were confirmed on the outside. The lesional veins drained into the inferior vermian vein [
Figure 4
Surgical view (a) and indocyanine green (ICG) videography (b-e) during the operation. The main feeding arteries (arrows) are first filled with ICG (b and c), followed by transit feeders (open arrowhead) and the simultaneous filling of drainers (open arrow) approximately 8 seconds after the main feeding arteries are visualized (d). Finally, the non-feeding arteries (arrowhead) are filled with ICG approximately 15 seconds after the main feeding arteries are visualized (e)
DISCUSSION
Hemangioblastomas are benign tumors (WHO I) comprising neoplastic stromal cells and abundant small vessels.[
In recent years, there have been several reports of presurgical embolization being performed in order to control bleeding during hemangioblastoma surgeries.[
Liu et al. compared the outcomes of groups in which presurgical embolization was or was not performed for cerebellar hemispheric hemangioblastomas. The authors reported that the duration of surgery was shortened, and the amount of blood loss and subsequent need for transfused blood were decreased in the embolization group.[
Hemangioblastomas are characterized by abundant blood flow and it has been strongly advocated that they should be removed en bloc.[
Hitherto, ICG has been employed routinely during surgery for brain aneurysms, cerebral arterial malformations, and bypass surgeries.[
However, the use of ICG also has its drawbacks. Deep blood vessels, covered by the cerebral parenchyma, cannot be visualized.[
We did not perform an embolization in either of these cases owing to concerns of the possibility of complications. Such complications could have been fatal (e.g., in Case #1) because of the proximity of the tumors to the brainstem. In such cases, we demonstrated that there is a way to use ICG without embolization. It seems that ICG can be successfully utilized by examining the surgical strategy with preoperative images.
CONCLUSION
For hypervascular tumors of the posterior fossa, preoperative image assessment is important. Furthermore, the use of ICG during surgery can be advantageous when the feeding arteries and draining veins exist superficially in the operative field and as a result, making tumor removal en bloc safer.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amano T, Tokunaga S, Shono T, Mizoguchi M, Matsumoto K, Yoshida T. Cerebellar hemangioblastoma manifesting as hearing disturbance. Neurol Med Chir (Tokyo). 2009. 49: 418-20
2. Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: Implications for treatment. J Neurosurg. 2006. 105: 248-55
3. Ampie L, Choy W, Lamano JB, Kesavabhotla K, Kaur R, Parsa AT. Safety and outcomes of preoperative embolization of intracranial hemangioblastomas: A systematic review. Clin Neurol Neurosurg. 2016. 150: 143-51
4. Cornelius JF, Saint-Maurice JP, Bresson D, George B, Houdart E. Hemorrhage after particle embolization of hemangioblastomas: Comparison of outcomes in spinal and cerebellar lesions.J. Neurosurg. 2007. 106: 994-8
5. Cui H, Zou J, Bao YH, Wang MS, Wang Y. Surgical treatment of solid hemangioblastomas of the posterior fossa: A report of 28 cases. Oncol Lett. 2017. 13: 1125-30
6. Eskridge JM, McAuliffe W, Harris B, Kim DK, Scott J, Winn HR. Preoperative endovascular embolization of craniospinal hemangioblastomas. AJNR Am J Neuroradiol. 1996. 17: 525-31
7. Hao S, Li D, Ma G, Yang J, Wang G. Application of intraoperative indocyanine green videoangiography for resection of spinal cord hemangioblastoma: Advantages and limitations. J Clin Neurosci. 2013. 20: 1269-75
8. Hojo M, Arakawa Y, Funaki T, Yoshida K, Kikuchi T, Takagi Y. Usefulness of tumour blood flow imaging by intraoperative indocyanine green videoangiography in hemangioblastoma surgery. World Neurosurg. 2014. 82: E498-E501
9. Katayama S, Kidoguchi K, Takeda N. Preoperative embolization of solid cerebellar hemangioblastomas using n-butyl-cyanoacrylate. JNET. 2013. 7: 286-93
10. Kim EH, Cho JM, Chang JH, Kim SH, Lee KS. Application of intraoperative indocyanine green videoangiography to brain tumour surgery. Acta Neurochir. 2011. 153: 1487-95
11. Liu AH, Peng TM, Wu Z, Xiao XR, Jiang CH, Wu ZX. Clinical effectiveness of preoperative embolization for cerebella hemangioblastoma. Asian Pac J Cancer Prev. 2013. 14: 5179-83
12. Louis DN, Ohaki H, Wiestler OD, Cavenee WK.editorsWHO classification of tumours of the central nervous system. Lyon: IART; 2016. p.
13. Nakamura H, Kuratsu J, Shuuin T. Craniospinal hemangioblastoma associated with Von Hippel-Lindau disease review. Jpn J Neurosurg (Tokyo). 2013. 22: 52-60
14. Sakamoto N, Ishikawa E, Nakai Y, Akutsu H, Yamamoto T, Nakai K. Preoperative endovascular embolization for hemangioblastoma in the posterior fossa. Neurol Med Chir. 2012. 52: 878-84
15. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998. 121: 561-79
16. Shiroma A, Nishimura M, Nagamine H, Miyagi T, Hokama Y, Watanabe T. Cerebellar contribution to pattern separation of human hippocampal memory circuits. Cerebellum. 2016. 15: 645-62
17. Takeshima Y, Tanaka Y, Hironaka Y, Shida Y, Nakase H. Visualization of vascular structure of spinal hemangioblastoma using intraoperative indocyanine green videoangiography and temporary feeder occlusion. Eur Spine J. 2015. 24: S585-9
18. Takeuchi S, Tanaka R, Fujii Y, Abe H, Ito Y. Surgical treatment of hemangioblastomas with presurgical endovascular embolization. Neuro Med Chir. 2001. 41: 246-52
19. Tamura Y, Hirota Y, Miyata S, Yamada Y, Adam T, Kuroiwa T. The use of intraoperative near-infrared indocyanine green videoangiography in the microscopic resection of hemangioblastomas. Acta Neurochir. 2012. 154: 1407-14
20. Ueba T, Abe H, Matsumoto J, Higashi T, Inoue T. Efficacy of indocyanine green videography and real-time evaluation by FLOW 800 in the resection of a spinal cord hemangioblastoma in a child. J Neurosurg Pediatrics. 2012. 9: 428-31
21. Ueba T. Application of intraoperative indocyanine green videography to brain tumour surgery. Jpn J Neurosurg. 2014. 23: 871-5
22. Yoshino M, Nakatomi H, Matsumoto J, Kin T, Saito T, Shono N. Usefulness of high-resolution 3D multifusion medical imaging for preoperative planning in patients with posterior fossa hemangioblastoma: Technical note. J Neurosurg. 2017. 127: 139-47