- Head, Department of Epilepsy Surgery, Neurological Center, Centro Médico ABC, Mexico City, Mexico
- Benemérita Universidad Autónoma de Puebla Medical School, Puebla, Mexico,
- Neurological Center, Centro Médico ABC, Mexico City, Mexico
- Department of Pediatric Neurology, Neurological Center, ABC Medical Center, Mexico City, Mexico.
Correspondence Address:
Enrique de Font-Réaulx, Head Department of Epilepsy Surgery, Neurological Center, Centro Médico ABC, Mexico City, Mexico.
DOI:10.25259/SNI_763_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Enrique de Font-Réaulx1, Andrea Solis-Santamaria2, Emilio Arch-Tirado3, Adalberto González-Astiazarán4. Thermosensitive/thermochromic silicone and infrared thermography mapping in 60 consecutive cases of epilepsy surgery. 01-Mar-2024;15:63
How to cite this URL: Enrique de Font-Réaulx1, Andrea Solis-Santamaria2, Emilio Arch-Tirado3, Adalberto González-Astiazarán4. Thermosensitive/thermochromic silicone and infrared thermography mapping in 60 consecutive cases of epilepsy surgery. 01-Mar-2024;15:63. Available from: https://surgicalneurologyint.com/surgicalint-articles/12781/
Abstract
Background: Epilepsy surgery represents a therapeutic opportunity for those patients who do not respond to drug therapy. However, an important challenge is the precise identification of the epileptogenic area during surgery. Since it can be hard to delineate, it makes it necessary to use auxiliary tools as a guide during the surgical procedure. Electrocorticography (ECoG), despite having shown favorable results in terms of reducing post-surgical seizures, have certain limitations. Brain mapping using infrared thermography mapping and a new thermosensitive/thermochromic silicone (TTS) in epilepsy surgery has introduced a new resource of noninvasive and real-time devices that allow the localization of irritative zones.
Methods: Sixty consecutive patients with drug-resistant epilepsy with surgical indications who decided to participate voluntarily in the study were included in the study. We measured brain temperature using two quantitative methods and a qualitative method: the TTS sheet. In all cases, we used ECoG as the gold standard to identify irritative areas, and all brain tissue samples obtained were sent to pathology for diagnosis.
Results: In the subgroup in which the ECoG detected irritative areas (n = 51), adding the results in which there was a correlation with the different methods, the efficiency obtained to detect irritative areas is 94.11% (n = 48/51, P ≤ 0.0001) while the infrared thermography mapping method independently has an efficiency of 91.66% (P ≤ 0.0001). The TTS has a sensitivity of 95.71% and a specificity of 97.9% (P ≤ 0.0001) to detect hypothermic areas that correlate with the irritative zones detected by ECoG. No postoperative infections or wound dehiscence were documented, so the different methodologies used do not represent an additional risk for the surgical proceedings.
Conclusion: We consider that the infrared thermography mapping using high-resolution infrared thermography cameras and the TTS are both accurate and safe methods to identify irritative areas in epilepsy surgeries.
Keywords: Electrocorticography, Epilepsy surgery, Infrared thermography mapping, Thermochromic silicone, Thermosensitive silicone
INTRODUCTION
Epilepsy surgery represents a therapeutic opportunity for those patients who do not respond to drug therapy or have seizure syndromes remediable by surgery. In some specific cases, such as focal epilepsy, it is the only potentially curative treatment.[
Penfield and Jasper first described the ECoG and it is used since then, for the delimitation of the epileptogenic network and the identification of the irritative zones[
The ECoG, despite having shown favorable results in terms of reduced freedom from post-surgical seizures, has certain limitations.[
Thermography is based on brain metabolism, where thermal fluctuations during different physiological and pathological processes have been described.[
In neurosurgery, cortical mapping of functional areas using infrared thermography has demonstrated high sensitivity and specificity, offering an advantage over other discontinuous methods for a sustained evaluation of activation sites simultaneously.[
Brain mapping of areas using infrared thermography in epilepsy surgery has introduced a new possibility of a noninvasive and real-time tool that allows the localization of irritative zones. Areas with cortical dysplasia with epileptogenic activity identified employing ECoG had lower temperature ranges than normal cerebral cortex and nonirritative dysplastic tissue, usually with a radial heating pattern, where the area of higher temperature is furthest from the irritative zone, with a temperature difference (ΔT) of 1.2– 7.1°C. The histopathological results obtained after resection corresponded with diagnoses of type II-A and III-A cortical dysplasia according to the classification of the International League Against Epilepsy (ILAE).[
In the previous studies, we evaluated a thermosensitive/thermochromic silicone (TTS) as a qualitative thermographic method. This material provides the observer with a thermal imprint of the contacted surface for 3–30 s, being reactive within a temperature range of 21–38°C. In an initial three-stage study, different methods based on thermographic mapping were comparatively tested, including using the TTS. In a series of 10 cases, a 100% correlation was reported between infrared thermography mapping (quantitative method), thermosensitive silicone (qualitative method), and ECoG in the detection of irritative zones in patients with drug-resistant temporal lobe epilepsy.[
MATERIALS AND METHODS
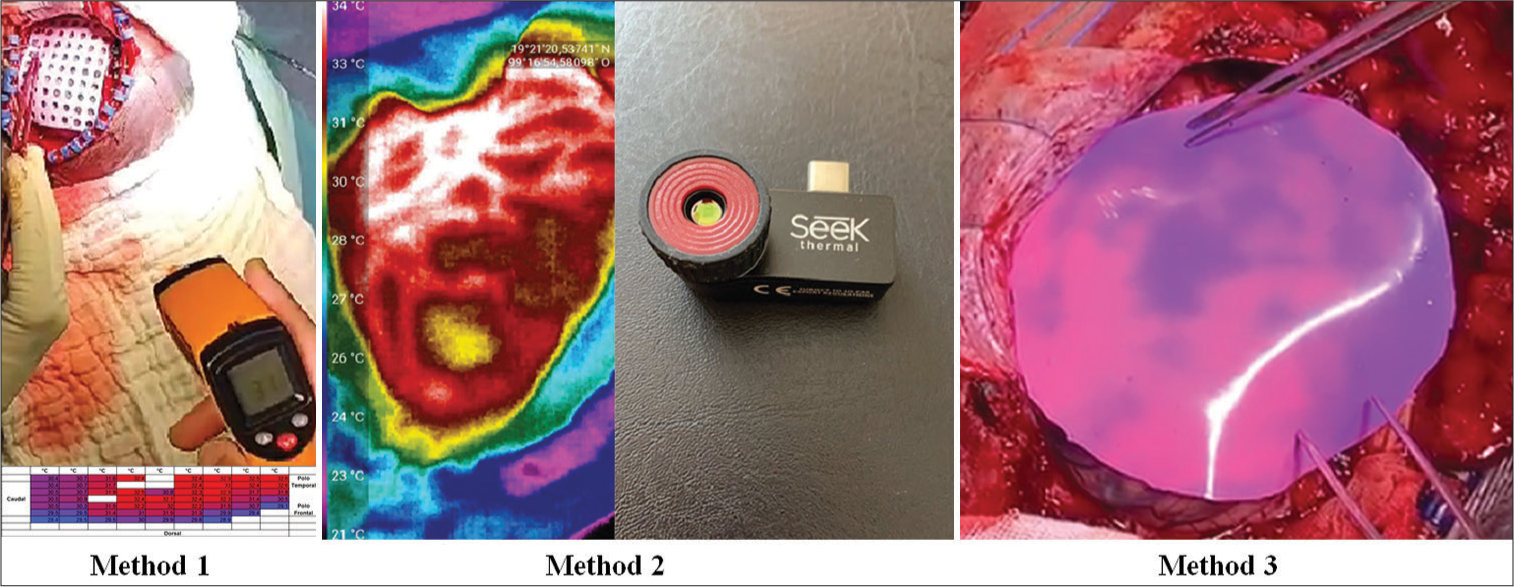
Consecutive patients with drug-resistant epilepsy with surgical indications who decided to participate voluntarily in the study were included in the study. We measured brain temperature using two quantitative methods: an infrared thermography camera (Seek Thermal®) and an infrared thermography thermometer with a laser pointer (Fluoreon®) and by a qualitative method: the TTS sheet (patent pending). The methodology of this study had three stages for measuring the electromagnetic radiation of the brain using thermography: In the first stage, we used a thermometer with a laser pointer, and the measurements obtained through a perforated silicone grid were recorded and emptied at an Excel table with color gradients, using blue for the coldest point and red for the hottest (Method 1); in the second stage, the use of a high-resolution infrared thermography camera that takes pictures and video was added to the previous methodology (Method 2) and in the third stage, we added the use of the TTS sheet (Method 3) [
Bivariate analyses and statistical tests were applied for correlation between the measured variables such as age, age at surgery, gender, chronicity of epilepsy, number of seizures per month, methodology used, type of surgery performed, thermographic pattern obtained, ECoG results, and pathology. The efficacy of the TTS to identify irritative areas identified by the gold standard, that is, ECoG, was analyzed separately.
Figure 1:
Examples of the three methods described. Method 1: Graph of brain’s surface temperatures recorded using the infrared thermometer with laser pointer, where color gradients were added, being red for the warmest point and blue for the coldest point of the recording. Method 2: Example of thermal gradients obtained by the high-resolution thermography camera (capable of taking photos and videos). Method 3: Example of the color contrast achieved by the thermosensitive/thermochromic silicone when in contact with the brain surface. Note that the purple basal color corresponds to the coldest part of the cerebral cortex and pink to the warmest areas, generating a thermal imprint that allows differentiating temperatures with the naked eye, without the need of filters, dyes, contrasts, or special lights.
RESULTS
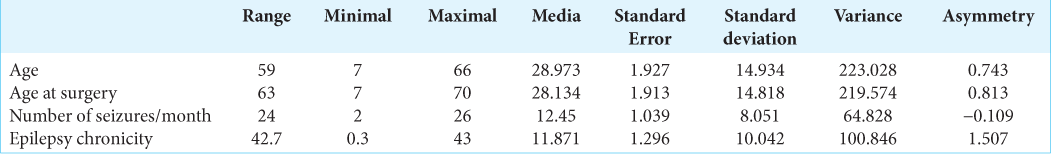
We included 60 patients, of which 33 were male (55%) and 27 were female (45%). The mean age of the study group was 28.97 years (±14.93 standard deviation), with a range of 7–66 years. The female average age was 27.82 years and 29.91 for the male, being no considerable variation concerning age and gender. The average seizures per month of the participants were 12.45 (±8.05 seizures/month) with a range of 2–26 seizures and with a modal interval of 17 seizures in 16 subjects (26.7%), two seizures in 11 subjects (18.3%), and three seizures six subjects (10%) and for the rest of the distribution, the frequency was 2 and 1 monthly seizures. The chronicity of epilepsy was 11.87 years (range 0.3–43 years, ±10.04) [
Diverse types of epilepsy surgeries by craniotomy were performed [
To analyze the average age and its variability concerning histopathology, an error graph was constructed for the distribution of these variables where it is observed that in ILAE type IIIA dysplasia, which was modal, the average age was 30.09 years. Similarly, the highest density interval was recorded in ILAE type III-D dysplasia with an average age of 23.1 years, so there are no correlations between age and histological results. In the last three values of the graph, there was no interval since there was only one subject for each variable [
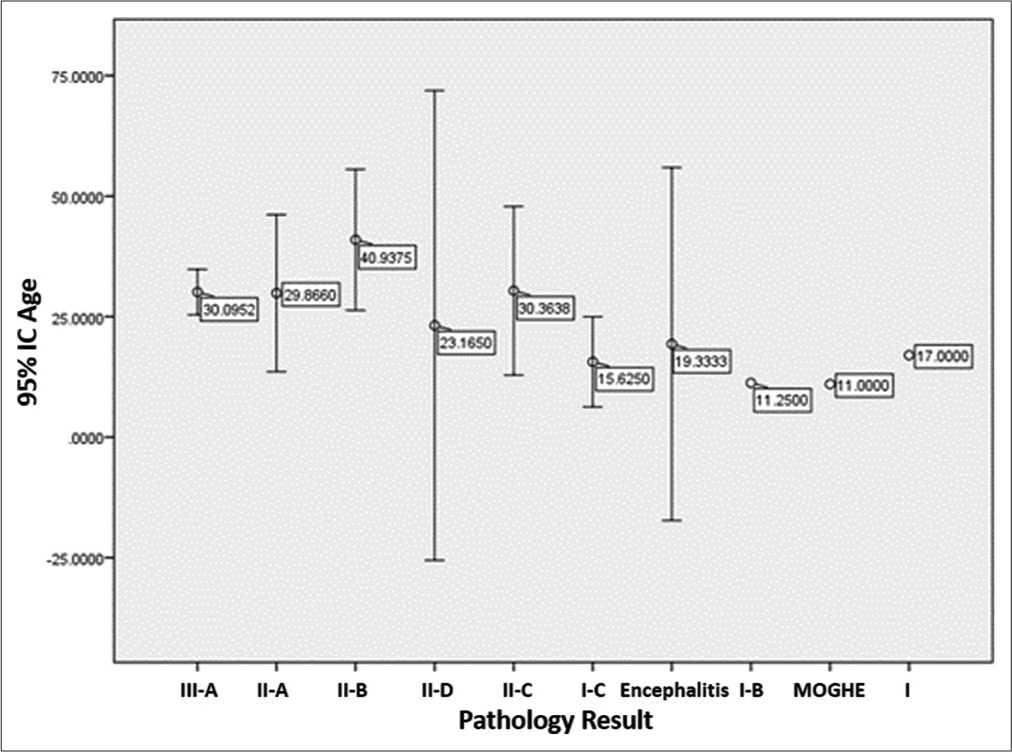
The Chi-square test was applied from the correlation contingency table versus the methodology, obtaining a significance of P ≤ 0.0001. In the subgroup in which the ECoG detected irritative areas (n = 51), adding the results in which there was a correlation with the different methods, the efficiency obtained to detect irritative areas is 94.11% (n = 48/51, P ≤ 0.0001) while the infrared thermography mapping method independently has an accuracy of 91.66% (P ≤ 0.0001) [
Graph 2:
Correlation between methodology 1, 2, and 3 and electrocorticography (ECoG) trivariate graphic. (A) Correlation between method 1, method 2, and ECoG; (B) correlation in two hypothermic regions detected with method 1 and 2 with irritative zones detected by ECoG regions; (C) correlation between method 1, 2, and 3 without irritative zones detected by ECoG; (D) only method 2 was used; and (E) correlation between method 1 and 2 in functional hemispherectomy cases. In selected cases, we added contralateral electroencephalography (EEG) in order to document electrical changes after interhemispheric disconnections.
In the subgroup (n = 47) that includes the use of the TTS designed to identify irritative areas due to their lower temperature compared to the rest of the exposed cerebral cortex (methodology 3), we compared its efficacy with the ECoG results, considered the gold standard. In 44 cases (93.61%), the detection of irritative areas by both methods coincided completely. In 1 case (2.1%), there was a partial correlation since the ECoG detected only one irritative area, and the TTS detected two areas that were colder than the rest of the cerebral cortex, both being within a dysplasia and in 2 cases (4.2%), there was no correlation between the methods. The TTS has a sensitivity of 95.71% and a specificity of 97.9% (P ≤ 0.0001) to detect hypothermic areas that correlate with the irritative zones detected by ECoG. The time required to make the infrared thermography mappings was 3 min, the TTS 2 min, and the ECoG was 35 min on average. The cost of infrared thermography and the TTS is much lower than that of ECoG.
We independently analyze whether the results of the methodologies used are affected by the number of crises per month or gender, ruling out this possibility. Applying the Chi-square test to the variables of the neuropathology and the methodologies used results, we conclude that the histological diagnosis does not affect the accuracy of infrared thermography mapping or the TTS.
A trivariate histogram was constructed between correlation, thermographic pattern, and epilepsy chronicity, to know the probabilistic intersection between these three variables based on the number of participants, where chronicity does not affect any trend.
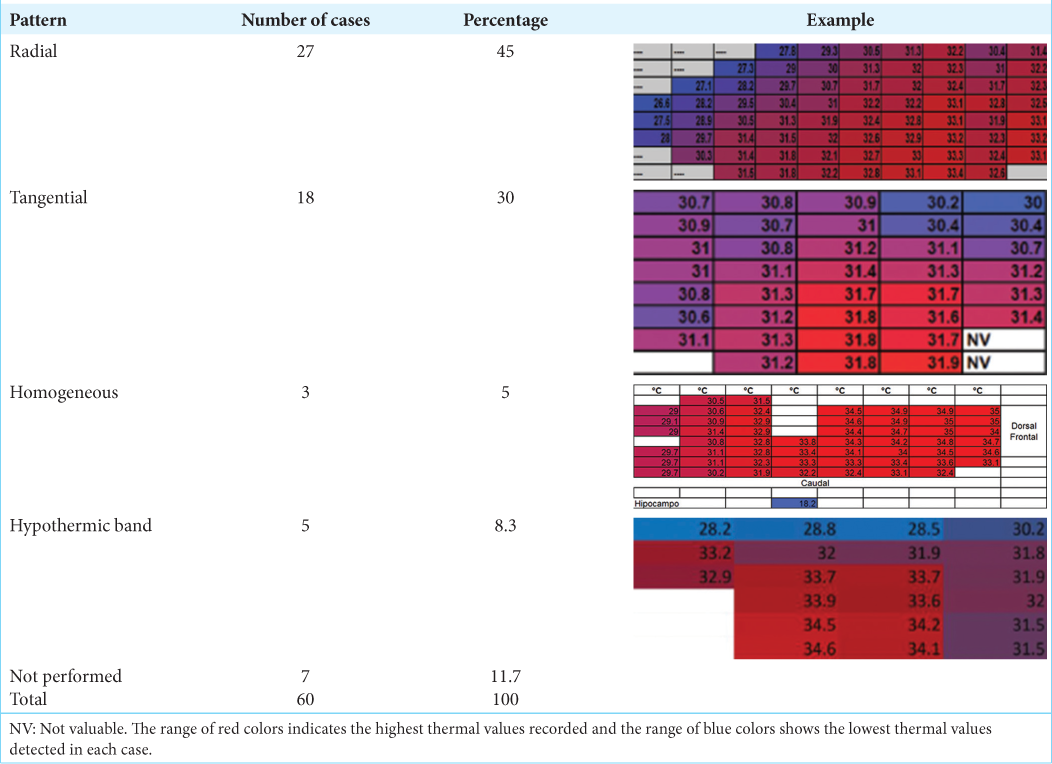
When analyzing the frequency and percentage of the thermographic pattern obtained, the modal frequency was radial with 27 cases (45%), followed by tangential with 18 cases (30%) [
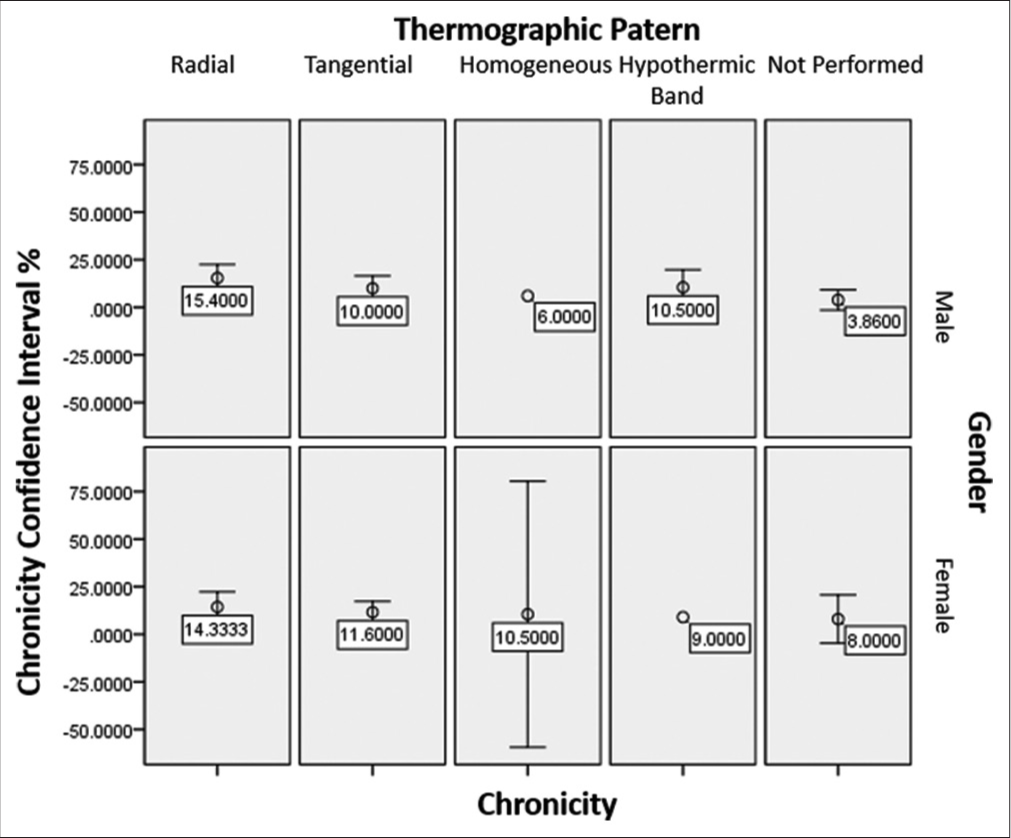
To analyze the thermographic patterns with the average chronicity, based on age and gender, an error bar graph was constructed. In the radial thermographic pattern concerning gender, the chronicity averages are constant at 15.4 years for males and 14.33 years for females, with very homogeneous distributions. Similar behavior was obtained in the tangential thermographic pattern where the average chronicity in the male was ten years, and in the female, it was 11.6 years, with very compact distributions [
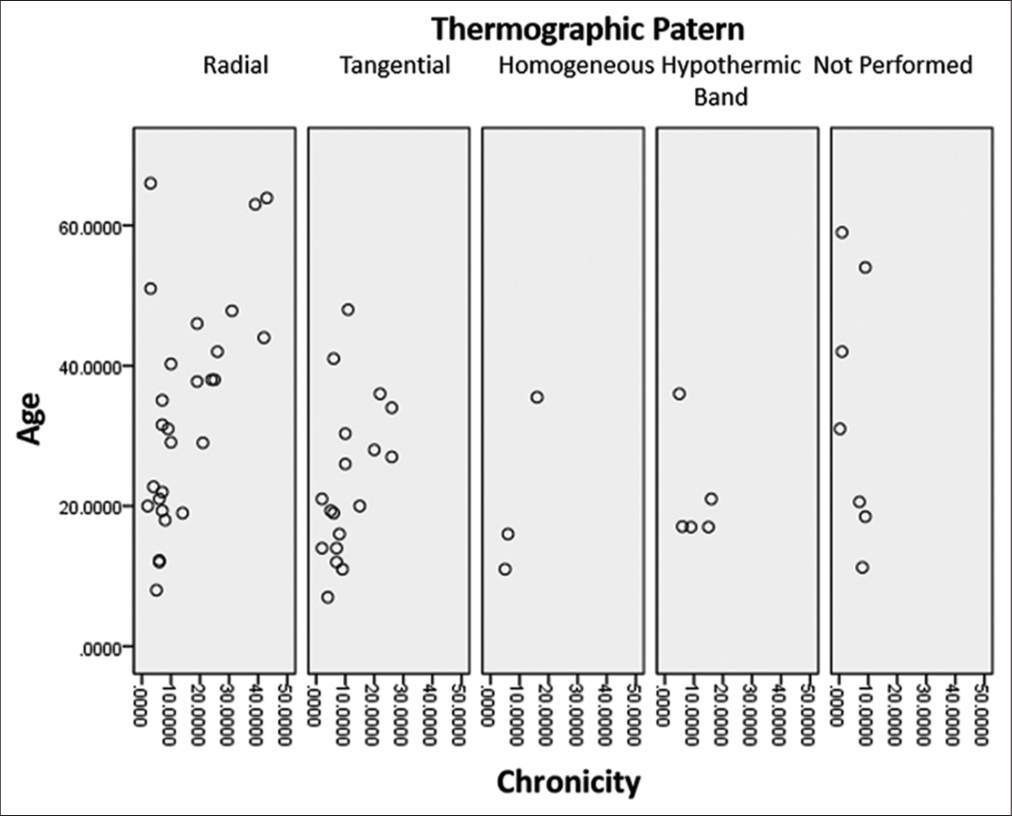
Independently, a scatter graph of the thermographic pattern and age based on chronicity was constructed, where it is observed that there is an increasing trend concerning age and chronicity in the radial and tangential thermographic patterns: As age increases, logically, the chronicity of epilepsy increases, drawing attention to the fact that the tangential thermographic pattern predominates in subjects under 50 years of age [
We did not have any postoperative infections or wound dehiscence, so the different methodologies used do not represent an additional risk for the surgical proceedings.
DISCUSSION
The success of epilepsy surgery depends on a delicate balance between disconnection, resection, or efficient inhibition of the epileptogenic mass on the one hand against the preservation of brain tissue to avoid permanent or disabling neurological deficits on the other hand. The challenge is even greater since it is almost impossible to establish these limits with the naked eye or even with the surgical microscope during surgeries. This justifies developing new methods to establish these surgical margins accurately and safely.
Our study population, despite having been diverse in their demographic and pathological variables, had no significant differences, so it represents an adequate study group to establish the usefulness of the different methodologies. We tested three different methods to identify irritative areas, which were compared against the gold standard that is ECoG. Both quantitative infrared thermography mapping (infrared cameras) and qualitative thermography (TTS) methods can identify different brain metabolic patterns due to their heat generation and, therefore, due to their electromagnetic radiation. In general, the ΔT is 94.11% correlated with the irritative areas identified by ECoG. Using infrared thermography mapping, four different thermal patterns were obtained, which could be correlated with the metabolic effect of epileptic seizures, and their study may open new lines of research on the pathophysiology of epilepsy.
Few hospitals have the infrastructure to perform ECoG in all epilepsy surgery cases, and in all health systems, it is very important to optimize resources. The TTS costs up to 50 times less than ECoG. Individually, the TTS has a sensitivity of 95.71% and a specificity of 97.9% (P ≤ 0.0001) to detect hypothermic areas that coincide with the irritative zones detected by ECoG.
As none of the thermography techniques used, caused any adverse effects, it can be stated that both infrared thermography mapping and the TTS are safe, with an efficacy comparable to ECoG to identify irritative areas, with a cost and time of application significantly less than the ECoG both in pediatric patients, as in adults, regardless of the chronicity and etiology of epilepsy.
CONCLUSION
We consider that the infrared thermography mapping using high-resolution infrared thermography cameras and the TTS are both accurate and safe methods to identify irritative areas in epilepsy surgeries, with the advantage that their application takes only 3 min for infrared thermography and a few seconds for TTS. Both methods have a significantly lower cost than ECoG. The infrared thermography mapping technique and the TTS can also be useful in other neurosurgical indications, in awake surgery, and in general surgery in humans and other species of animals.
Ethical approval
Approved on July, 24th, 2019, Number: ABC-19-39 by our Institutional Ethics Committee and by our Institutional Review Board at the Centro Medico ABC, Mexico.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
The principal author may apply for a patent of the STT described.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alsaaod M, Büscher W. Detection of hoof lesions using digital infrared thermography in dairy cows. J Dairy Sci. 2012. 95: 735-42
2. Boran E, Sarnthein J, Krayenbühl N, Ramantani G, Fedele T. High-frequency oscillations in scalp EEG mirror seizure frequency in pediatric focal epilepsy. Sci Rep. 2019. 9: 16560
3. Bousselham A, Bouattane O, Youssfi M, Raihani A. Towards reinforced brain tumor segmentation on MRI images based on temperature changes on pathologic area. Int J Biomed Imaging. 2019. 2019: 1758948
4. Cardone D, Trevisi G, Perpetuini D, Filippini C, Merla A, Mangiola A. Intraoperative thermal infrared imaging in neurosurgery: Machine learning approaches for advanced segmentation of tumors. Phys Eng Sci Med. 2023. 46: 325-37
5. Cepeda C, Levinson S, Nariai H, Yazon VW, Tran C, Barry J. Pathological high frequency oscillations associate with increased GABA synaptic activity in pediatric epilepsy surgery patients. Neurobiol Dis. 2020. 134: 104618
6. De Font-Réaulx E, Lluch JT, López RL, Bialik PS, Corona MÁ, López LG. Thermography mapping patterns in temporal lobe epilepsy surgery. Surg Neurol Int. 2020. 11: 30
7. De Font-Réaulx E, Terrazo-Lluch J, Díaz-López LG, ColladoCorona MÁ, Shkurovich-Bialik P, González-Astiazarán A. Localization of irritative zones in epilepsy with thermochromic silicone. Surg Neurol Int. 2022. 13: 14
8. Demuru M, Kalitzin S, Zweiphenning W, van Blooijs D, Van’t Klooster M, Van Eijsden P. The value of intra-operative electrographic biomarkers for tailoring during epilepsy surgery: From group-level to patient-level analysis. Sci Rep. 2020. 10: 14654
9. Fernández IS, Loddenkemper T. Electrocorticography for seizure foci mapping in epilepsy surgery. J Clin Neurophysiol. 2013. 30: 554-70
10. Gorbach AM, Heiss J, Kufta C, Sato S, Fedio P, Kammerer WA. Intraoperative infrared functional imaging of human brain. Ann Neurol. 2003. 54: 297-309
11. Goel K, Pek V, Shlobin NA, Chen JS, Wang A, Ibrahim GM. Clinical utility of intraoperative electrocorticography for epilepsy surgery: A systematic review and meta-analysis. Epilepsia. 2023. 64: 253-65
12. Greiner HM, Horn PS, Tenney JR, Arya R, Jain SV, Holland KD. Preresection intraoperative electrocorticography (ECoG) abnormalities predict seizure-onset zone and outcome in pediatric epilepsy surgery. Epilepsia. 2016. 57: 582-9
13. Gorbach AM, Heiss JD, Kopylev L, Oldfield EH. Intraoperative infrared imaging of brain tumors. J Neurosurg. 2004. 101: 960-9
14. Hoffmann KP, Ruff R, Kirsch M. SEP-induced activity and its thermographic cortical representation in a murine model. Biomed Tech. 2013. 58: 217-23
15. Murugesan S, Bouchard K, Chang E, Dougherty M, Hamann B, Weber GH. Multi-scale visual analysis of time-varying electrocorticography data via clustering of brain regions. BMC Bioinform. 2017. 18: 236
16. Naydenov E, Minkin K, Penkov M, Nachev S, Stummer W. Infrared thermography in surgery of newly diagnosed glioblastoma multiforme: A technical case report. Case Rep Oncol. 2017. 10: 350-5
17. Ravat S, Iyer V, Panchal K, Muzumdar D, Kulkarni A. Surgical outcomes in patients with intraoperative Electrocorticography (EcoG) guided epilepsy surgery-experiences of a tertiary care centre in India. Int J Surg. 2016. 36: 420-8
18. Roessler K, Heynold E, Buchfelder M, Stefan H, Hamer HM. Current value of intraoperative electrocorticography (iopECoG). Epilepsy Behav. 2019. 91: 20-4
19. Sadeghi-Goughari M, Mojra A, Sadeghi S. Parameter estimation of brain tumors using intraoperative thermal imaging based on artificial tactile sensing in conjunction with artificial neural network. J Phys D Appl Phys. 2016. 49: 075404
20. Steiner G, Sobottka SB, Koch E, Schackert G, Kirsch M. Intraoperative imaging of cortical cerebral perfusion by time-resolved thermography and multivariate data analysis. J Biomed Opt. 2011. 16: 016001
21. Sugano H, Shimizu H, Sunaga S. Efficacy of intraoperative electrocorticography for assessing seizure outcomes in intractable epilepsy patients with temporal-lobe-mass lesions. Seizure. 2007. 16: 120-7
22. Suzuki T, Oishi N, Fukuyama H. Simultaneous infrared thermal imaging and laser speckle imaging of brain temperature and cerebral blood flow in rats. J Biomed Opt. 2018. 24: 031014
23. Tripathi M, Garg A, Gaikwad S, Bal CS, Chitra S, Prasad K. Intra-operative electrocorticography in lesional epilepsy. Epilepsy Res. 2010. 89: 133-41
24. Zweiphenning W, van’t Klooster MA, van Klink NE, Leijten FS, Ferrier CH, Gebbink T. Intraoperative electrocorticography using high-frequency oscillations or spikes to tailor epilepsy surgery in the Netherlands (the HFO trial): A randomised, single-blind, adaptive non-inferiority trial. Lancet Neurol. 2022. 21: 982-93








Adalberto González Astiazarán
Posted March 2, 2024, 9:20 am
Deeply appreciate your approval for published our work.