- Department of Neurosurgery, Universidade Federal de São Paulo, São Paulo, Brazil.
Correspondence Address:
Guilherme Salemi Riechelmann, Department of Neurosurgery, Universidade Federal de São Paulo, São Paulo, Brazil.
DOI:10.25259/SNI_446_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel Tau Zymberg, Guilherme Salemi Riechelmann, Marcos Devanir Silva da Costa, Clauder Oliveira Ramalho, Sergio Cavalheiro. Third ventricle colloid cysts: An endoscopic case series emphasizing technical variations. 27-Jul-2021;12:376
How to cite this URL: Samuel Tau Zymberg, Guilherme Salemi Riechelmann, Marcos Devanir Silva da Costa, Clauder Oliveira Ramalho, Sergio Cavalheiro. Third ventricle colloid cysts: An endoscopic case series emphasizing technical variations. 27-Jul-2021;12:376. Available from: https://surgicalneurologyint.com/surgicalint-articles/10994/
Abstract
Background: Colloid cyst treatment with purely endoscopic surgery is considered to be safe and effective. Complete capsule removal for gross total resection is usually recommended to prevent recurrence but may not always be safely feasible. Our objective was to assess the results of endoscopic surgery using mainly aspiration and coagulation without complete capsule resection and discuss the rationale for the procedure.
Methods: A retrospective review was conducted of 45 consecutive symptomatic patients with third ventricle colloid cysts that were surgically treated with purely endoscopic surgery from 1997 to 2018.
Results: Mean age was 35.4 years. Male-to-female ratio was 1:1. Clinical presentation included predominantly headache (80%). Transforaminal was the most used route (71.1%) followed by transeptal (24.5%) and interforniceal (4.4%). Capsule was intentionally not removed in 42 patients (93.3%) and cyst remnants were absent on postoperative MRI in 36 (85%). Mild complications occurred in 8 patients (17.8%). Surgery was statistically associated with cyst volume and ventricular size reduction. There were no serious complications, shunts or deaths. Follow-up did not show any recurrence or remnant growth that needed further treatment.
Conclusion: Gross total resection may not be the main objective for every situation. Subtotal resection without capsule removal seems to be safer while preserving good results, especially in a limited resource environment. Remnants left behind should be followed but tend to remain clinically asymptomatic for the most part. Surgical planning allows the surgeon to choose among the different resection routes and techniques available. Decisions are predominantly based on preoperative imaging and intraoperative findings.
Keywords: Capsule remnant, Colloid cyst, Endoscopic surgery, Gross total resection
INTRODUCTION
Colloid cysts (CCs) are a relatively rare benign cystic lesion originating from brain endodermal embryonic remnants, most commonly occurring in the rostral portion of the third ventricle, immediately adjacent to the interventricular Foramen of Monro (FM). Symptomatic patients usually present with obstructive hydrocephalus due to a blockage of cerebrospinal fluid (CSF) pathways around the FM.[
Higher rates of gross total resection (GTR) reported on the microsurgical series are associated with increased morbidity and lower recurrence.[
The endoscopic approach is a well-established surgical option for excision of CC.[
The endoscopic view permitted a better understanding of CC’s attachments to the roof of the third ventricle and its relations to the surrounding structures such as the fornix, deep venous system, FM, choroid plexus, and septum pellucidum (SP). Based on anatomical knowledge, preoperative radiological evaluation and intraoperative findings, surgical planning and techniques for intraventricular endoscopic surgery may vary.[
Surgeons often use the transforaminal (TF) route for the most usual location of the CC at the FM. It consists in reaching the cyst, opening its capsule, aspirating its mucin content, and coagulating or resecting its capsule through the FM. Nevertheless, there are situations in which route variations such as the transseptal (TS) or interforniceal (IF) are necessary to properly aspirate, coagulate, and/or remove the CC.
In this paper, the authors reviewed a series of endoscopic cases since the beginning of our learning curve. Surgical planning, intraoperative decision-making, and technique variations are emphasized.
MATERIALS AND METHODS
A retrospective review of 45 CC patients who were operated between 1997 and 2018 was performed. Ethical approvals of the institutions where the patients were treated and signed informed consent statements were obtained. It was done according to the preferred reporting of case series in surgery guideline.[
All patients submitted to endoscopic surgery for third ventricle CC excision with at least a 1-year postoperative follow-up met inclusion criteria. Patients with <1 year of follow-up or with insufficient records were excluded from the study.
Surgery was indicated for patients with neurological symptoms due to a well-documented CC in the third ventricle on MRI. All patients had enlarged ventricles and clinical intracranial hypertension. The diagnosis was confirmed by anatomopathological examination.
Every procedure was performed with a neuroendoscope system (FF370 R, Aesculap or Decq, Karl Storz) with a rigid 0° telescope by endoscopic experienced neurosurgeons (STZ, SC). Pre- and postoperative ventricular system dimensions were analyzed using the Evans index. The maximal width of the frontal horns was divided by the maximal width of the inner table of the cranium at the level of the frontal horn.
Volumetric analysis of CCs before and after surgery was performed to quantify the degree of residual disease using Osirix (Pixmeo, Switzerland). Paper printed MRIs were excluded from the analysis.
Quantitative variables were compared using the Wilcoxon matched-pairs signed-rank test; α = 0.05 was considered indicative of statistical significance. Data were analyzed, and graphs were created with Prism 8 software for Mac OS, version 8.4.3 (GraphPad Software, San Diego, California, USA).
Radiological evaluation and surgical planning
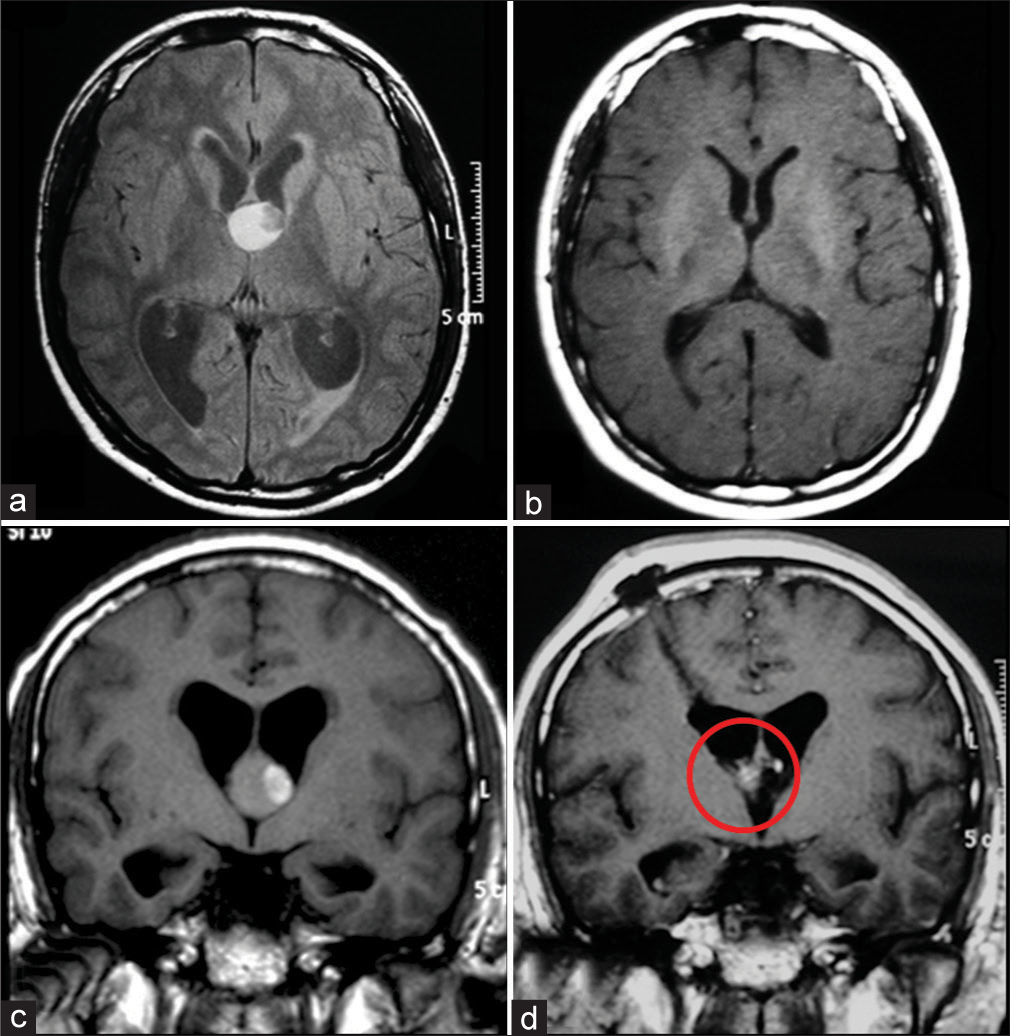
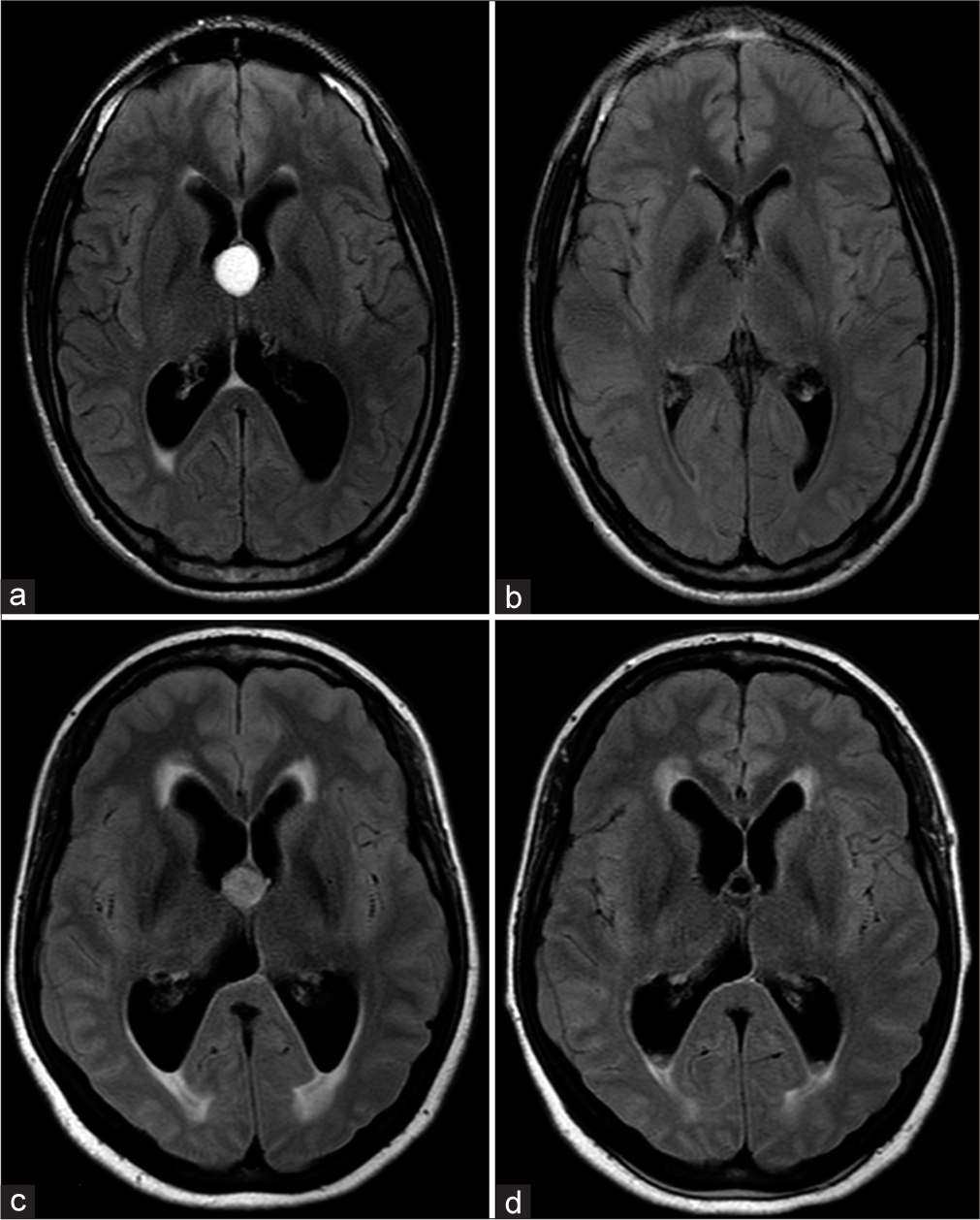
Even though CCs are most easily seen on CT due to their spontaneous hyperdensity, MRI is necessary for a proper evaluation. MR signal can vary on T1, T2, or even FLAIR sequences and the CC may not be equally visible in all of them. On T1, half have a high signal while the rest is iso- or hypointense to the adjacent brain. On T2, most have a homogeneous low signal, some a low signal in the center with high signal on its margins and a few have a homogeneously high signal. Low T2 signal CCs are difficult to see on FLAIR due to a signal that resembles CSF [
Some key parameters must be appreciated for surgical planning. The viscosity of CCs content can be indirectly estimated by how low the T2 signal is, especially when combined with high T1 signal.[
CCs relation to the FM and its size is also relevant. When big enough, it can protrude itself through the FM reaching the lateral ventricle. Mass effect can also reduce or close the ipsilateral FM. This is the reason we decided to measure CC according to its lateral dimensions.
Some can originate not in the rostral but in the retroforaminal portion of the third ventricle instead. These CCs, when cavum septi pellucidi (CSP) and cavum vergae are present, tend to grow superiorly above the roof of the third ventricle due to an inexistence of the superior midline barrier caused by a detachment of the fornices. Their position in between SP leaflets may require a different technique than the usual TF.
The ventricular system is usually dilated due to obstructive hydrocephalus in symptomatic patients. If the lateral ventricles are asymmetric, an entry through the larger one tends to be safer and easier [
The choroid plexus and vascular anomalies of the septal, thalamostriate, or the internal cerebral veins can be visualized and have to be minded in order to avoid intraventricular hemorrhage.
Standard operative technique
Anesthesia
All patients were put under general anesthesia through an endotracheal tube and intravenous medication.
Positioning
All surgeries were done with the patient in a supine position. The head was placed slightly flexed without any rotation over a gel donut head positioner and was elevated around 25°.
Side
The right side was mostly chosen to avoid approach-related trauma to the dominant hemisphere. The left side was an option when: (1) the left ventricle was significantly larger than the right one as seen on preoperative imaging, (2) the left FM was wider, and (3) the CC protruded itself through the left FM.
Burr hole
The placement of the burr hole just laterally to Kocher’s point was done as standard practice. After a frontoparietal longitudinal skin incision of about 30 mm over the coronal suture, we placed the burr hole 3 cm laterally to the midline and 1 cm anteriorly to the coronal suture.
Ventricular cannulation
All of our patients had a dilated ventricular system. Therefore, reaching the lateral ventricle with the endoscope did not require neuronavigation in any case. A perpendicular entry angle on the cortex was enough to reach the lateral ventricle.
Intraventricular inspection
After the ventricular system was reached, anatomical aspects could be observed. At this point, the surgeon was guided by visual parameters and neuronavigation was not necessary.
First, we’ve analyzed the FM size if it was too small for manipulation, deformed, or compressed, how much of the cyst was visible through it and if there were any choroid plexus over it that needed coagulation. Note that in most patients, the FM was in its regular shape and size with the choroid plexus by its entrance.
The SP was then studied. It was important to check for its integrity, for the presence of a CSP or a bulging over it. The bulging indicates a cyst in between the leaflets, covered by the septum.
TF route
The majority of CCs were treated using TF route. It consists of the manipulation of the lesion through the FM. We did it when the CC was located in the anterior part of the third ventricle, which was the majority of them. If the CC was in the posterior part of the third ventricle or was big enough to reach high above its roof, another technique was required for the CC to be properly accessed (TSR or IFR).
The CC was often covered by choroid plexus, which had to be coagulated until it shrank enough to expose the entire cyst. The capsule was opened on its central region with scissors or forceps and the mucin content was aspirated using a 6F Fogarty catheter. We did not coagulate the cyst before content aspiration to avoid thicker and denser content.
After complete aspiration, the remaining capsule was meticulously inspected and coagulated. Frequently, a saline solution was slightly forced inside the cyst to expel the colloid material outside, which was also aspirated when visible in the ventricular system. Septostomy was routinely performed for all patients for a better distribution of CSF.
Careful manipulation was necessary to avoid fornix contusion. When no important structures were attached to the capsule or when dissection was feasible, complete resection was accomplished with mild rotatory traction. Injury to ventricular structures as the FM caused by endoscope compression can damage the anterior column of the fornix, anterior thalamic tubercle, and the genu of the internal capsule, resulting in permanent neurological impairment. Lesions of the choroid plexus or the ventricular veins result in serious hemorrhages that can be managed with continuous irrigation for long-lasting periods until it fully stops [
Figure 3:
Transforaminal route. CCs aspiration and capsule coagulation through the left: (a) Panoramic view of the left lateral ventricle, (b) choroid plexus coagulation and capsule opening, (c) content aspiration 4-F catheter, (d) complete aspiration, loose capsule, (e) capsule coagulation, note attached choroid plexus, (f) final CP coagulated, (g) complete absence of lesion at endoscopic inspection, (h) panoramic at the end of procedure: free third ventricle, septostomy in place. CC: Colloid cyst, CP: Choroid plexus, SV: Septal vein, TSV: Thalamostriate vein, F: Fornix, IIIVT: Third ventricle, SPT: Septostomy, FM: Foramen of Monro.
TS route
When CSP was present, CC grew in between the leaflets of the SP causing a bulging, too high and posterior to be reached through the FM. In these situations, the leaflet of the SP over the CC had to be opened on the superior part of the bulge so the capsule could be accessed. An opening in the inferior part of the bulge can increase the risk of damage to the fornix.
Once the CC had been reached, the rest of the technique for opening, aspiration, and coagulation was essentially the same [
IF route
This approach was chosen when the IF raphe was split by a posterior CC itself or by the presence of a CSP. The CC was clearly prominent posteriorly between the two crura of the fornices in these cases and the SP was usually stretched and perforated by chronic hydrocephalus. The manipulation of the endoscope is facilitated by a wider area due to the window between the two crura that exist in these patients [
Figure 5:
Interforniceal route: (a) Right lateral ventricle view. Red circle detail in (a), (b) coagulation and section septum pellucidum, posterior septal vein. (c) Posterior colloid cyst protruding between fornices. (d) Inspection after removal of all cyst content. (e) Coagulation of all membrane leading to shrinkage.
RESULTS
Purely endoscopic surgery results were analyzed for the 45 patients who met inclusion criteria. Procedures took place between 1997 and 2018. There were 23 male individuals and 22 were female. The mean age was 35.4 years, the youngest was 13 years old, and the oldest, 72 years old.
Clinical presentation included predominantly headache (80%). Other symptoms were less frequently observed such as cognitive impairment (20%), drop attacks (20%), vomiting (6.66%), hearing impairment (4.44%), visual impairment (2.22%), and vertigo (2.22%). All patients were symptomatic at presentation and none of the CCs was incidentally diagnosed.
Patients did not have any focal neurological deficits and most of them had no decreased level of consciousness before surgery. One patient was previously treated with a ventriculoperitoneal (VP) shunt for hydrocephalus and was operated on in an emergency due to its failure. A shunt was not necessary after surgery.
Procedures were always purely endoscopic. The CCs were aspirated and coagulated. Aspiration without coagulation was done in one patient. For most of the cases, the capsule was coagulated and its removal was not attempted to reduce surgical complications.
Rarely CC was located posteriorly between the two crura of the fornices or in between the SP leaflets. Hence, TF was the most used route (71.1%) followed by TS (24.5%) and IF (4.4%).
The right side was chosen in 41 patients (91.1%) while the left side was preferred in 4 (8.9%). The first 14 cases were sent to the intensive care unit for postoperative care for precaution. Then, with more experience and operative time reduction, all of our patients were extubated in the surgical theater and sent to the ward.
Although the capsule was intentionally not removed in 42 patients (93.3%), cyst remnants were absent on postoperative MRI in 36 of those patients (85%). On the other hand, GTR, which includes the complete removal of the capsule, was performed on 3 patients (6.7%) [
The mean cyst volume before surgery was of 1.8 cm3 (max 3.8 and min 0.5). Postoperatively, cyst remnants were absent on MRI in 93% (39 patients) considering both GTR and nonGTR. For the other 7%, no cyst remnant had a volume higher than 0.15 cm3 [
Surgical procedure and the postoperative period were uneventful for 32 patients (71.1%). Vomiting and/or nausea in the first 24 h were a frequent feature easily controlled with antiemetic drugs. Mild complications occurred in 8 patients (17.8%). Cyst bleeding after its puncture was seen in only 1 patient (2.2%), which was easily managed with continuous irrigation.
Mild meningeal symptoms were referred by 3 patients (6.7%) on the 1st day after the procedure without any severe consequences and complete improvement on the next days. Mild ventricular hemorrhage appeared incidentally on postoperative imaging in 2 patients (4.4%), both asymptomatic. Fornix contusion occurred in 2 patients (4.4%) and no memory deficit developed. All those events are classified as Grade I in the Clavien-Dindo classification.[
CSF flow was reestablished with the CC aspiration and coagulation alone for all patients. CCs rest were stable for non-GTR patients, all of them asymptomatic [
External ventricular drainage was not used in any patient nor intracranial pressure monitoring device was used. There were no cases of late hydrocephalus or need for a VP shunt. Ventricular dimensions at the end of the follow-up period were smaller than before surgery in all patients. Preoperative mean Evans index was 0.35 and postoperative, 0.29 (max 0.45 min 0.3) [
Two of our patients required two surgical procedures for adequate cyst evacuation in the same hospital stay. Both were right in the beginning of our series and the surgical route initially chosen was not the best suited for each case.
DISCUSSION
We found that although non-GTR was achieved in 42 (93.33%) patients, remnants were identified in only 9 (20%) on postoperative MRI, and only 2 (4.4%) required retreatment. These two patients came from the beginning of the series within the group in which only aspiration was performed.
Since the beginning of our series and experience, a single working channel endoscope was used. For a safer procedure, we have chosen to aspirate the mucin content and coagulate the capsule without completely removing it from the adherences to the third ventricle walls and vascular structures around it. Surgery was associated with a ventricular size and cyst volume reduction in a statistically significant fashion.
Fornix contusions, as shown by Oertel et al., are the most common type of intraoperative injury in endoscopic ventricular surgery. It occurs most often when operating lesions in the posterior part of the third ventricle. They are usually small contusions and are not correlated with any symptoms in most of these patients.[
Surgical steps and indications can vary widely since CC is a rare benign lesion with no randomized clinical trials. Decision-making is, thus, highly related to surgeons practice. Due to the risk of acute deterioration or even sudden death, symptomatic patients must be treated.[
Janjua et al. described a guideline to CCs management based on expert experience and uses a modified colloid cyst risk scoring (mCCRS) to determine therapy. All cases must be followed since 5–15% of asymptomatic patients will need surgery for the next 5 years due to neurological deterioration.[
The analysis of our results showed that, for most cases, even with intraoperative non-GTR, postoperative MRI will most likely appear as if a GTR was performed, with no visible remnants [
Most of our patients that had the capsule leftover, despite the absence of visible remnants on MRI, still have a clear MRI with no recurrence. The ones with cyst remnants on postoperative MRI until now did not have any enlargement of cyst dimensions. None of the patients became symptomatic a 2nd time nor needed to be reoperated.
GTR rates vary widely across literature with good results in non-GTR series. In Hellwig et al., most patients had cyst remnants but only one needed a reoperation 1 year later.[
When we observe carefully the series in which GTR was attempted for all patients, more serious complications were present despite good outcomes for most cases. Samadian et al. reported permanent hemiparesis, mortality, and the need for EVD and VP shunts.[
Severe complications might not have been adequately valued or emphasized in some of the papers that recommend GTR. Janjua et al.[
Isaacs et al. considered that the endoscopic CC surgery without complete capsule removal is a viable option for successfully treating CCs of the third ventricle.[
In the opposite, according to Vorbau et al., Levine et al., and Teo, GTR should be the goal of CC endoscopic surgery because remnants, although predominantly asymptomatic, can cause future problems.[
For Vorbau et al., sophisticated endoscopic equipment and sharp dissection technique can make GTR safely achievable.[
Even though we consider that a gross total removal is probably the ideal objective and that the partial resection technique used does not replace it for most cases, we have seen that the sole coagulation of the capsule without its removal is actually effective in terms of prognosis, symptoms relief, and cyst recurrence. It is more accessible and affordable while associated with lower morbidity rates.
CONCLUSION
Purely endoscopic surgery for third ventricle CCs can be considered a first-line therapy for endoscope experienced neurosurgeons.
Surgical planning allows the surgeon to choose among the different resection routes and techniques available. Decisions are predominantly based on preoperative imaging and intraoperative findings.
GTR may not be the main objective for every situation. Subtotal resection without capsule removal seems to be safer while preserving good results, especially in a limited resource environment. Remnants left behind should be followed but tend to remain clinically asymptomatic for the most part.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agha RA, Borrelli MR, Farwana R, Koshy K, Fowler AJ, Orgill DP. The PROCESS 2018 statement: Updating consensus preferred reporting of case series in surgery (PROCESS) guidelines. Int J Surg. 2018. 60: 279-82
2. Armao D, Castillo M, Chen H, Kwock L. Colloid cyst of the third ventricle: Imaging-pathologic correlation. AJNR Am J Neuroradiol. 2000. 21: 1470-7
3. Azab WA, Najibullah M, Yosef W. Endoscopic colloid cyst excision: Surgical techniques and nuances. Acta Neurochir (Wien). 2017. 159: 1053-8
4. Boogaarts HD, Decq P, Grotenhuis JA, Le Guerinel C, Nseir R, Jarraya B. Long-term results of the neuroendoscopic management of colloid cysts of the third ventricle: A series of 90 cases. Neurosurgery. 2011. 68: 179-87
5. Brostigen CS, Meling TR, Marthinsen PB, Scheie D, Aarhus M, Helseth E. Surgical management of colloid cyst of the third ventricle. Acta Neurol Scand. 2017. 135: 484-7
6. Brun A, Egund N. The pathogenesis of cerebral symptoms in colloid cysts of the third ventricle: A clinical and pathoanatomical study. Acta Neurol Scand. 1973. 49: 525-35
7. Burhan Janjua M, Reddy S, El Ahmadieh TY, Ban VS, Hwang SW, Ozturk AK. Inchoate guidelines of endoscopic resection of colloid cysts. J Clin Neurosci. 2020. 71: 1-8
8. Connolly ID, Johnson E, Lamsam L, Veeravagu A, Ratliff J, Li G. Microsurgical vs. endoscopic excision of colloid cysts: An analysis of complications and costs using a longitudinal administrative database. Front Neurol. 2017. 8: 259
9. Decq P, Le Guerinel C, Brugières P, Djindjian M, Silva D, Kéravel Y. Endoscopic management of colloid cysts. Neurosurgery. 1998. 42: 1288-94
10. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. 240: 205-13
11. Doron O, Feldman Z, Zauberman J. MRI features have a role in pre-surgical planning of colloid cyst removal. Acta Neurochir (Wien). 2016. 158: 671-6
12. El-Ghandour NM. Endoscopic treatment of third ventricular colloid cysts: A review including ten personal cases. Neurosurg Rev. 2009. 32: 395-402
13. Haider G, Laghari AA, Shamim MS. Choosing between endoscopic or microscopic removal of third ventricle colloid cysts. J Pak Med Assoc. 2017. 67: 1458-9
14. Hellwig D, Bauer BL, Schulte M, Gatscher S, Riegel T, Bertalanffy H. Neuroendoscopic treatment for colloid cysts of the third ventricle: The experience of a decade. Neurosurgery. 2008. 62: 1101-9
15. Hoffman CE, Savage NJ, Souweidane MM. The significance of cyst remnants after endoscopic colloid cyst resection: A retrospective clinical case series. Neurosurgery. 2013. 73: 233-7
16. Horn EM, Feiz-Erfan I, Bristol RE, Lekovic GP, Goslar PW, Smith KA. Treatment options for third ventricular colloid cysts: Comparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007. 60: 613-8
17. Isaacs AM, Bezchlibnyk YB, Dronyk J, Urbaneja G, Yong H, Hamilton MG. Long-term outcomes of endoscopic third ventricle colloid cyst resection: Case series with a proposed grading system. Oper Neurosurg (Hagerstown). 2020. 19: 134-42
18. Kornienko VN, Pronin IN.editors. Diagnostic Neuroradiology. Berlin: Springer; 2008. p.
19. Levine NB, Miller MN, Crone KR. Endoscopic resection of colloid cysts: Indications, technique, and results during a 13-year period. Minim Invasive Neurosurg. 2007. 50: 313-7
20. Lewis AI, Crone KR, Taha J, van Loveren HR, Yeh HS, Tew JM. Surgical resection of third ventricle colloid cysts. Preliminary results comparing transcallosal microsurgery with endoscopy. J Neurosurg. 1994. 81: 174-8
21. Marx S, Schroeder HW. Endoscopic bimanual sharp dissection technique for gross-total resection of colloid cysts: Technical note. J Neurosurg. 2020. p. 1-9
22. Mishra S, Chandra PS, Suri A, Rajender K, Sharma BS, Mahapatra AK. Endoscopic management of third ventricular colloid cysts: Eight years’ institutional experience and description of a new technique. Neurol India. 2010. 58: 412-7
23. Oertel J, Linsler S, Emmerich C, Keiner D, Gaab M, Schroeder H. Results of combined intraventricular neuroendoscopic procedures in 130 cases with special focus on fornix contusions. World Neurosurg. 2017. 108: 817-25
24. Samadian M, Ebrahimzadeh K, Maloumeh EN, Jafari A, Sharifi G, Shiravand S. Colloid cyst of the third ventricle: Long-term results of endoscopic management in a series of 112 cases. World Neurosurg. 2018. 111: e440-8
25. Sethi A, Cavalcante D, Ormond DR. Endoscopic versus microscopic transcallosal excision of colloid cysts: A systematic review in the era of complete endoscopic excision. World Neurosurg. 2019. 132: e53-8
26. Sribnick EA, Dadashev VY, Miller BA, Hawkins S, Hadjipanayis CG. Neuroendoscopic colloid cyst resection: A case cohort with follow-up and patient satisfaction. World Neurosurg. 2014. 81: 584-93
27. Teo C. Complete endoscopic removal of colloid cysts: Issues of safety and efficacy. Neurosurg Focus. 1999. 6: e9
28. Vorbau C, Baldauf J, Oertel J, Gaab MR, Schroeder HW. Long-term results after endoscopic resection of colloid cysts. World Neurosurg. 2019. 122: e176-85
29. Yadav YR, Yadav N, Parihar V, Kher Y, Ratre S. Management of colloid cyst of third ventricle. Turk Neurosurg. 2015. 25: 362-71