- Geisel School of Medicine, Section of Neurological Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA
- Departement of Surgery, Section of Neurological Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA
- Departement of Pathology, Section of Neurological Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA
Correspondence Address:
Vyacheslav Makler
Departement of Surgery, Section of Neurological Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA
DOI:10.25259/SNI-90-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Derrek Schartz, Erin D’Agostino, Vyacheslav Makler, William F. Hickey, David F. Bauer. Third ventricle World Health Organization Grade II meningioma presenting with intraventricular hemorrhage and obstructive hydrocephalus: A case report and literature review. 24-Apr-2019;10:73
How to cite this URL: Derrek Schartz, Erin D’Agostino, Vyacheslav Makler, William F. Hickey, David F. Bauer. Third ventricle World Health Organization Grade II meningioma presenting with intraventricular hemorrhage and obstructive hydrocephalus: A case report and literature review. 24-Apr-2019;10:73. Available from: http://surgicalneurologyint.com/surgicalint-articles/9280/
Abstract
Background:Third ventricular meningiomas are exceedingly rare intracranial tumors that may present with intraventricular hemorrhage.
Case Description:The patient is 46-year-old who initially presented with obstructive hydrocephalus from a presumed vascular lesion and who was treated with endoscopic third ventriculostomy. He presented 3 years later with acute intraventricular hemorrhage and hydrocephalus. The hemorrhage was evacuated and the third ventricular tumor was resected, and the patient made an excellent recovery. Histopathological analysis identified the tumor as the World Health Organization Grade II meningioma.
Conclusion:Third ventricular meningioma is a rare tumor that may present with hemorrhage and obstructive hydrocephalus. Surgical resection can be helpful for this rare presentation of intracranial meningioma.
Keywords: Endoscopic third ventriculostomy, intraventricular hemorrhage, intraventricular meningioma, obstructive hydrocephalus, World Health Organization grade II meningioma
INTRODUCTION
Intraventricular meningiomas are rare primary brain tumors, representing only 0.5–5% of all meningiomas.[
CASE report
The patient is 46-year-old with a past medical history significant for diverticulosis who initially presented with acute obstructive hydrocephalus requiring placement of an external ventricular drain (EVD). Magnetic resonance imaging (MRI) at the time revealed a small enhancing lesion in the posterior third ventricle at the cerebral aqueduct causing obstruction [
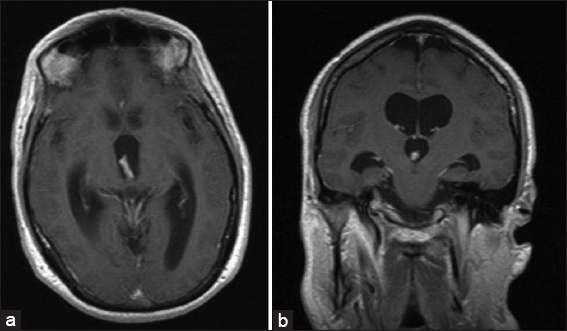
Figure 1
Magnetic resonance imaging from initial presentation with hydrocephalus (HCP). (a) T1-weighted image (T1WI) with contrast axial view. Demonstrates uniformly enhancing lesion within the posterior aspect of the third ventricle obstructing aqueduct. This results in triventricular HCP. (b) T1WI with contrast coronal views. Demonstrates uniformly enhancing mass within the cortical aspect of the third ventricle blocking the aqueduct. This results in triventricular HCP. No transependymal flow was noted on FLAIR sequence, suggesting HCP to be longstanding.
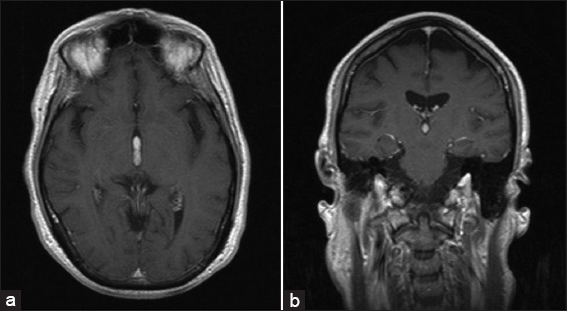
Figure 2
Last magnetic resonance imaging (MRI) before admission. (a) T1WI with contrast axial view (similar cut as initial MRI). Significant improvement in obstructive HCP following successful ETV. The uniformly enhancing mass within the third ventricle is stable in size compared to prior MRI. (b) T1WI with contrast coronal view (similar cut as initial MRI). The uniformly enhancing mass within the third ventricle appears to be stable in size, and spans almost the entire length of the third ventricle.
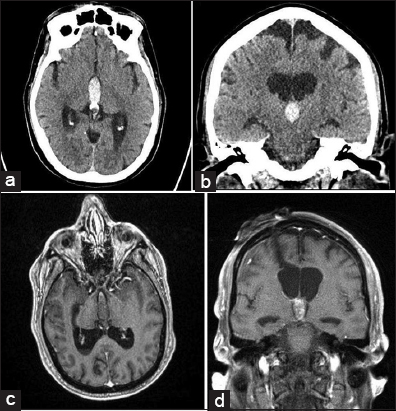
Figure 3
(a) CTH and magnetic resonance imaging during this admission. (a) Axial noncontrast head computed tomography (CT). IVH is noted within the third ventricle and aqueduct. Triventricular obstructive HCP is also noted. (b) Coronal non-contrast CTH. IVH is noted within the third ventricle which results in obstructive triventricular HCP. (c) T1WI with contrast axial view. IVH is noted within the third ventricle and occipital horns with resultant triventricular obstructive HCP. Contrast-enhancing lesion is noted within the anterior superior aspect. (d) T1WI with contrast coronal view. IVH is noted within the third ventricle with resultant triventricular obstructive HCP. The contrast-enhancing lesion is noted within the anterior superior aspect of the third ventricle.
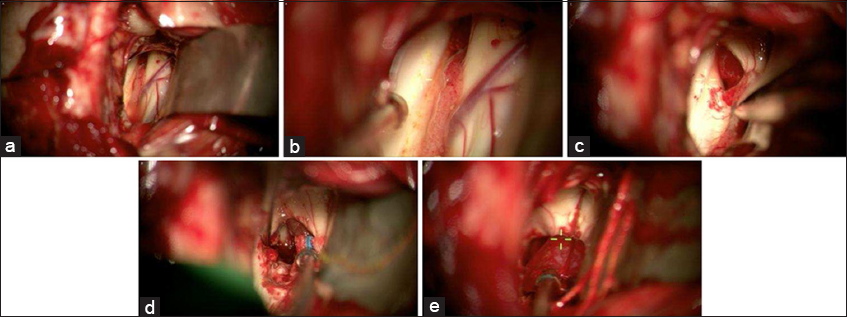
Figure 4
Intraoperative pictures of right sided interhemispheric transcallosal approach to the third ventricular mass. (a) Zoomed out view of the left lateral ventricle with foramen of Monro at 12 o’ clock, anterior septal vein at 3 o’ clock, and choroid plexus at the center of the picture. (b) zoomed in view of the left lateral ventricle with better visualization of the foramen of Monro, anterior septal vein, and choroid plexus. The red/purplish tumor is starting to come into view within the foramen of Monro. (c) The third ventricular tumor is better seen within the foramen of Monro. It appeared to be more red and vascular, as supposed to the IVH clot which looked dark purple. (d) The tumor is better visualized at the 1 o’clock position. (e) The tumor being removed. After the tumor was removed, the third ventricle was explored, and the IVH clot was noted to be more posterior. It was then suctioned out.
Figure 5
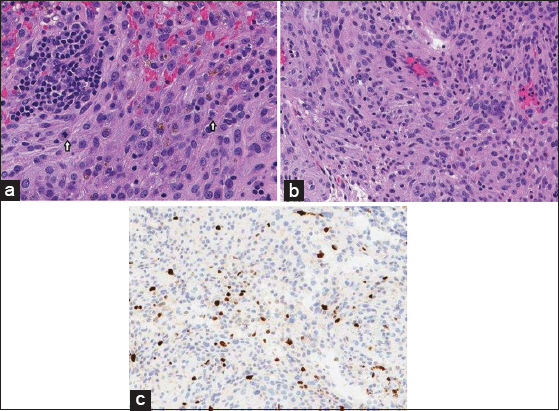
Histopathology of resected tumor specimen concluded to be a WHO Grade II meningioma. (a) The neoplasm is composed of pleomorphic cells with irregular nuclei and prominent nucleoli. Hemosiderin pigment is present in the center of the field, indicating prior hemorrhage. Two mitoses (white arrows) are present in this high magnification photomicrograph. (H and E, ×300). (b) The tumor does not have the morphology of a low-grade meningioma, but there is an attempt at whorl formation in the bottom left in this field. (H and E, ×250). (c) The Ki67 (Mib-1) nuclear labeling index is significantly higher that would be expected in low grade meningiomas. (Ki67 immunohistochemistry, ×125).
To put this case into the appropriate context of the available literature, we performed a systematic literature search and analyzed comparable cases.
Methods
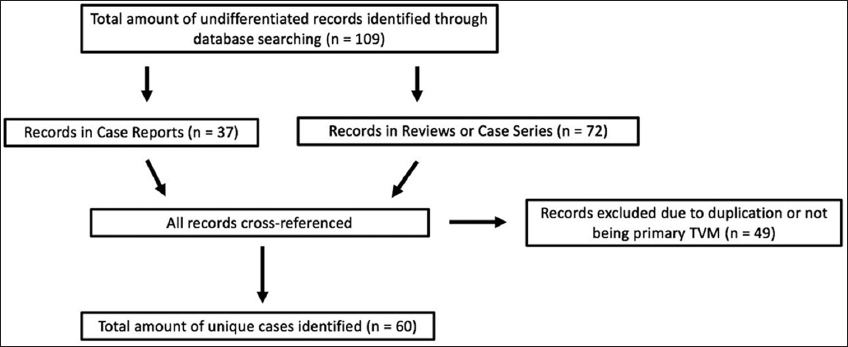
A PubMed search up to December 2018 using the key phrase “TVMs” returned 137 papers. From this initial search, we identified individual papers that included new cases of TVM and case series of intraventricular meningiomas that had TVM reports within them. This allowed us to identify 109 cases who had been reported in the literature. However, after carefully reviewing all of the identified records, we noticed that some reviews and case series had inadvertently duplicated prior reports when determining the total amount of cases of TVM in the literature. As a result, we cross-referenced all of the identified records and did not include reports based on the following exclusion criteria: if the case had been previously reported literature or if the TVM was not primary and had originated from a lateral ventricle or elsewhere. Doing this, we identified 60 unique cases of primary TVM in the literature (
We also wished to investigate and quantify the surgical approach across these studies and correlate them with outcomes. We found that the outcomes across the records were highly variable in how they were reported. As a result, we pooled together outcomes that were described as “good” - describing a range from simple survival to independent functioning - into an “optimal” category. Similarly, we pooled together outcomes described as “bad” - describing a range from death to dependent functioning - into a “suboptimal” category. We then constructed comparative tables detailing the outcomes. We additionally collected data across all 60 cases describing the patient characteristics and symptomatology of TVM.
RESULTS
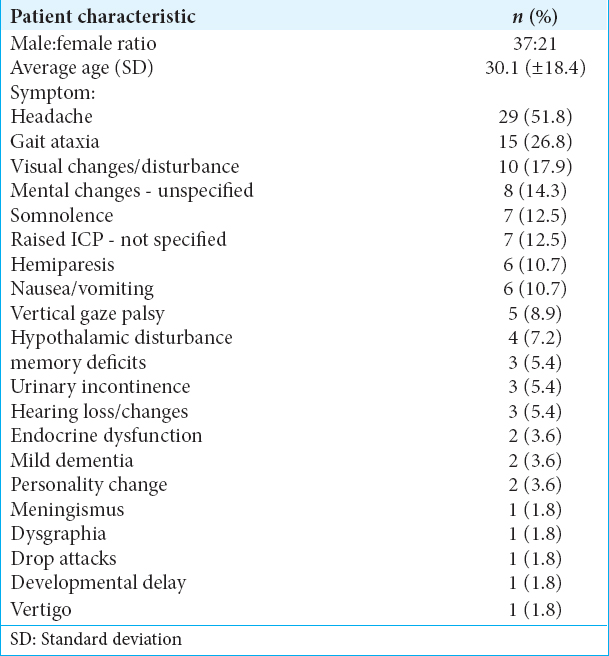
The patient characteristics and symptomatology of TVMs were evaluated across the 60 reviewed cases. 37 patients were male and 21 were female (1.76 male/1.0 female ratio), and in two cases, the sex was not reported. The average presenting age was 30.1 years old (Standard deviation=±18.4). The most common presenting symptoms were headache in 29 cases (51.8%), followed by gait ataxia in 15 cases (26.8%) and visual changes or disturbance in 10 cases (26.8%). Hypothalamic changes, including the development of diabetes insipidus, were a symptom in only 4 cases (7.2%). Various other neurologically related symptoms were also found and are detailed in
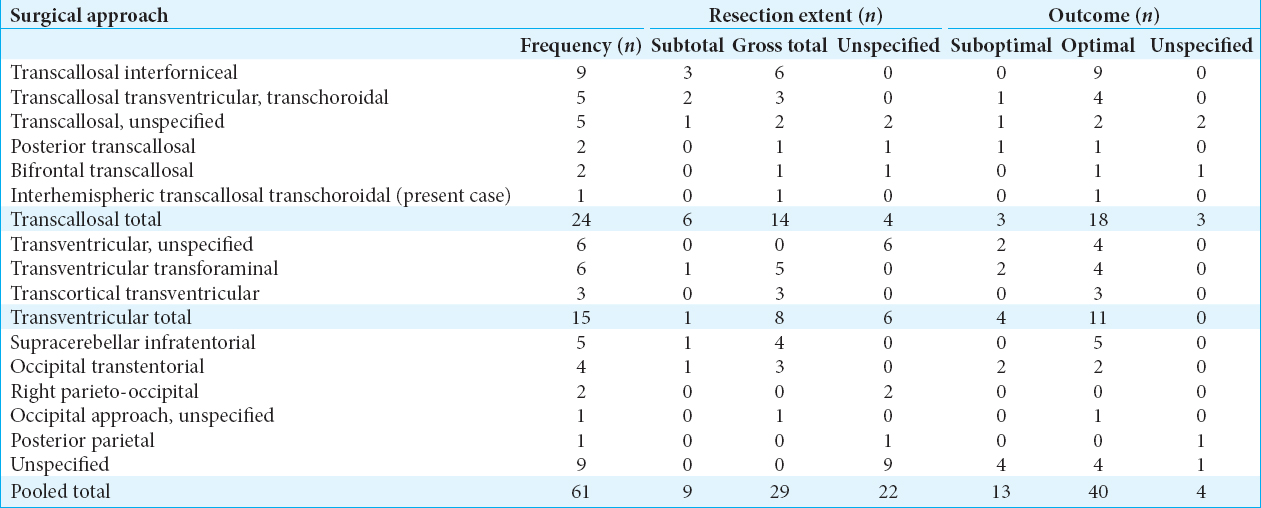
Surgical approaches for the treatment of TVMs were also investigated, along with the associated resection extent and outcomes for each technique. The frequency of use, resection extent (gross total vs. subtotal), and associated outcomes for each surgical approach of TVM are described and reported in
Transventricular approaches were the second most common surgical approach employed (24.6% of cases). The transventricular transforaminal approach was the most common specific transventricular technique used (9.8%, 4/6 success rate). A transventricular, unspecified approach (9.8%, 4/6 success rate) was also reported in 6 cases. This was followed by the transcortical transventricular approach (4.9%, 3/3 success rate).
Several other approaches were also utilized and described within the literature with varying frequency and success rates, including the supracerebellar infratentorial approach (8.2%, 5/5 success rate) and occipital transtentorial approach (6.6%, 2/4 success rate), among other approaches that are described in
DISCUSSION
TVMs are rare tumors. To date, 60 unique cases have been reported in the literature. Most cases are reported in isolated case reports or case series describing all types of intraventricular meningiomas.[
The third ventricle is an abnormal location for meningiomas to be found because the third ventricle lacks a dural attachment. TVMs are postulated to arise from the tela choroidea of the velum interpositum.[
On histopathological analysis, the resected tumor consisted of sheets of pleomorphic cells with prominent nucleoli and frequent mitoses. Structures typically associated with meningiomas (e.g., cellular whorls, psammoma bodies, and nuclear pseudoinclusions) were absent. The mitotic rate exceeded 4 per 10 high power fields, thus designating it as the WHO Grade II meningioma [
On literature review, the surgical approaches and management of TVM were investigated. We found that the transcallosal approach was the most commonly utilized approach across all 61 identified cases, including the present case. Interestingly, the transcallosal interforniceal approach was the most consistently successful and also the most commonly used overall [
Our patient presented with an initial episode of symptomatic HCP 3 years before the second hemorrhagic event. He was initially treated with ETV, but we refrained from a biopsy because the tumor appeared to be highly vascular and the patient was clinically nonfocal (other than the hydrocephalic presentation). There are also no previously documented instances of TVM presenting with hemorrhage after being monitored for years with serial imaging, which lead us to follow the lesion expectantly instead of resecting it. Ultimately, our patient underwent interhemispheric transcallosal transchoroidal approach with resultant gross total resection of the tumor after presenting with IVH. We chose this approach because the fornices were adherent to each other in our patient, making the interforniceal approach challenging. Our patient presented with IVH after several years of stable imaging. Surgery was not pursued earlier because of the risks associated with surgical tumor resection in a mostly neurologically intact patient. We would recommend serial imaging follow-up at 3 months, 6 months, and annually. Patients should be informed that hemorrhage is possible even if tumor remains stable in size. Without tissue diagnosis, we do not recommend stereotactic radiosurgery as the primary treatment modality.
CONCLUSION
TVM is an uncommon neurologic tumor. It most commonly presents with symptoms of increased intracranial pressure, ataxia, hemiparesis, or visual disturbance. Infrequently, it may present with IVH. Surgical management can be successfully achieved with a transcallosal approach.
Disclosures
None.
IRB: Per Dartmouth-Hitchcock Medical Center, IRB was not required for single patient case report.
Financial support and sponsorship
Section of Neurological Surgery, Department of Surgery, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
Conflicts of interest
There are no conflicts of interest.
References
1. Avman N, Dinçer C. Meningiomas of the third ventricle. Acta Neurochir (Wien). 1978. 42: 217-24
2. Bhatoe HS, Singh P, Dutta V. Intraventricular meningiomas:A clinicopathological study and review. Neurosurg Focus. 2006. 20: E9-
3. Bloomgarden GM, Byrne TN, Spencer DD, Heafner MD. Meningioma associated with aneurysm and subarachnoid hemorrhage:Case report and review of the literature. Neurosurgery. 1987. 20: 24-6
4. Cabezudo JM, Vaquero J, García-de-Sola R, Areitio E, Bravo G. Meningioma of the anterior part of the third ventricle. Acta Neurochir (Wien). 1981. 56: 219-31
5. Champagne PO, Bojanowski MW. Meningioma of the superior leaflet of the velum interpositum:A case report. Surg Neurol Int. 2015. 6: S132-5
6. Criscuolo GR, Symon L. Intraventricular meningioma. A review of 10 cases of the national hospital, queen square (1974-1985) with reference to the literature. Acta Neurochir (Wien). 1986. 83: 83-91
7. Dash C, Pasricha R, Gurjar H, Singh PK, Sharma BS. Pediatric intraventricular meningioma:A series of six cases. J Pediatr Neurosci. 2016. 11: 193-6
8. De la Sayette V, Rivaton F, Chapon F, Hubert P, Ganem F, Houtteville JP. Meningioma of the third ventricle. Computed tomography and magnetic resonance imaging. Neuroradiology. 1991. 33: 354-6
9. Grujicic D, Cavallo LM, Somma T, Illic R, Milicevic M, Raicevic S. Intraventricular meningiomas:A series of 42 patients at a single institution and literature review. World Neurosurg. 2017. 97: 178-88
10. Gruszkiewicz J, Doron Y, Gellei B, Peyser E. Massive intracerebral bleeding due to supratentorial meningioma. Neurochirurgia (Stuttg). 1969. 12: 107-11
11. Helle TL, Conley FK. Haemorrhage associated with meningioma:A case report and review of the literature. J Neurol Neurosurg Psychiatry. 1980. 43: 725-9
12. Ito J, Kadekaru T, Hayano M, Kurita I, Okada K, Yoshida Y. Meningioma in the tela choroidea of the third ventricle:CT and angiographic correlations. Neuroradiology. 1981. 21: 207-11
13. Jin BZ, Yuan GY, Yue SZ, Zhou X, Guan QK, Xu DW. The use of transcallosal-interforniceal approach for microsurgical removal of the third ventricle tumors. J Neurosurg Sci. 2015. 59: 19-24
14. Karki P, Yonezawa H, Bohara M, Oyoshi T, Hirano H, Moinuddin FM. Third ventricular atypical meningioma which recurred with further malignant progression. Brain Tumor Pathol. 2015. 32: 56-60
15. Kothbauer P, Jellinger K, Falment H. Primary brain tumour presenting as spontaneous intracerebral haemorrhage. Acta Neurochir (Wien). 1979. 49: 35-45
16. Li P, Diao X, Bi Z, Hao S, Ren X, Zhang J. Third ventricular meningiomas. J Clin Neurosci. 2015. 22: 1776-84
17. Lozier AP, Bruce JN. Meningiomas of the velum interpositum:Surgical considerations. Neurosurg Focus. 2003. 15: E11-
18. Nakamura M, Roser F, Bundschuh O, Vorkapic P, Samii M. Intraventricular meningiomas:A review of 16 cases with reference to the literature. Surg Neurol. 2003. 59: 491-503
19. Pereira BJ, de Almeida AN, Paiva WS, de Aguiar PH, Teixeira MJ, Marie SK. Natural history of intraventricular meningiomas:Systematic review. Neurosurg Rev. 2018. 2018: 1-11
20. Prickett JT, Klein BJ, Busch CM, Cuoco JA, Apfel LS, Marvin EA. Rapidly expanding lateral ventricular meningioma presenting with intraventricular hemorrhage following remote whole brain radiation and stereotactic radiosurgery. J Neurol Surg Rep. 2018. 79: e14-8
21. Strenger SW, Huang YP, Sachdev VP. Malignant meningioma within the third ventricle:A case report. Neurosurgery. 1987. 20: 465-8
22. Tung H, Apuzzo M.editors. Meningiomas. Meningiomas of the Third Ventricle and Pineal Region. New York: Raven Press Ltd; 1991. p. 583-91
23. Wajima D, Iida J, Nishi N. Third ventricular meningioma-case report. Neurol Med Chir (Tokyo). 2011. 51: 75-8
24. Winkler PA, Ilmberger J, Krishnan KG, Reulen HJ. Transcallosal interforniceal-transforaminal approach for removing lesions occupying the third ventricular space:Clinical and neuropsychological results. Neurosurgery. 2000. 46: 879-88