- Department of Surgery, Royal Victoria Infirmary, Newcastle-upon-Tyne, UK,
- Department of Neuropathology, James Cook University Hospital, Middlesbrough, UK,
- Department of Radiology James Cook University Hospital, Middlesbrough, UK,
- Department of Oncology, James Cook University Hospital, Middlesbrough, UK,
- Department of Oncology, University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom,
- Department of Neurosurgery, Bezmialem Vakif University Hospital, Istanbul, Turkey.
Correspondence Address:
Dr. Georges Sinclair, Consultant Oncologist, Department of Oncology, University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom.
DOI:10.25259/SNI_1065_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Callum Martin Allison1, David Scoones2, Arun Batra3, Georges Sinclair4,5,6. Thirteen-year long-term follow-up in a rare case of anaplastic astroblastoma: What makes the difference?. 27-May-2022;13:221
How to cite this URL: Callum Martin Allison1, David Scoones2, Arun Batra3, Georges Sinclair4,5,6. Thirteen-year long-term follow-up in a rare case of anaplastic astroblastoma: What makes the difference?. 27-May-2022;13:221. Available from: https://surgicalneurologyint.com/surgicalint-articles/11622/
Abstract
Background: Astroblastomas are uncommon neuroepithelial tumors of the central nervous system with a distinct, yet, controversial radiological, histological, and molecular profile. Debatable differences between low- and high-grade astroblastoma have been reported in the medical literature; indeed, despite the increasing relevance of molecular genetic profiling in the realm of astroblastoma, its application is still in its early stages. As a result, the diagnostic criteria for astroblastoma remain undecided with yet no real consensus on the most ideal management.
Case Description: This report describes a case of astroblastoma diagnosed 13 years ago in a young woman who despite six episodes of recurrence, transformation, and progression was able to retain a perfomace status of 0 by World Health Organization standard, throughout.
Conclusion: This report discusses the clinical, radiological, histological features, and management of this rare tumor with an extraordinarily long survival, with an aim to strengthen the literature on management options. To the best of our knowledge, this is the longest surviving case of anaplastic astroblastoma reported in the available medical literature.
Keywords: Astroblastoma, Histomolecular diagnosis, Long-term follow up, Neuro-oncology, Prognosis
INTRODUCTION
Astroblastomas are scarce and controversial primary neuroepithelial tumors of the central nervous system (CNS) occuring in the cerebral hemispheres of children and young adults with an incidence from 0.45% to 2.8% of all neuroglial tumors.[
As a result of a paucity of evidence on management, both low- and high-grade astroblastomas remain associated with a high degree of mortality. Moreover, with astroblastoma grading being a contentious issue due to the specific definition criteria, the OS of each grade is infrequently reviewed. In general terms, low-grade tumors have an OS in the region of years, whereas, high-grade or anaplastic tumors have been associated with a particularly poor prognosis, with the majority of cases not surviving beyond 1 year.[
CASE PRESENTATION
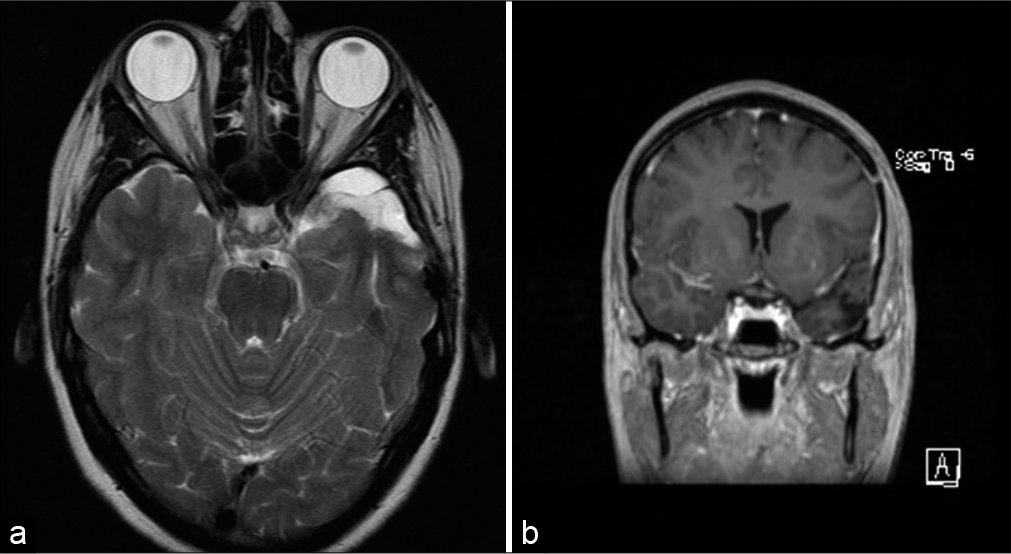
The authors present the case of a 40-year-old female who initially presented 13 years previously (October 2008) at the age of 27 years. The patient was 6-month postpartum complaining of progressive left-sided headaches. Initial magnetic resonance imaging (MRI) on T1-weighted series demonstrated a 5.5 cm × 3.9 cm × 4.8 cm left frontotemporal peripherally located mass with solid and cystic components with evidence for internal hemorrhage but relatively little surrounding parenchymal T2 hyperintensity [
Figure 1:
(October 2008): axial T2-weighted (a) and sagittal T1-weighted (b) images show a supratentorial peripherally located left frontotemporal lobulated fairly well-defined heterogeneous mass having cystic (thick arrows) and solid components with evidence of hemorrhage (thin arrows), and relatively little adjacent peritumoral parenchymal T2 hyperintensity (arrow heads). Axial (c) and coronal (d) postcontrast T1-weighted images reveal heterogeneous enhancement of the tumor with rim enhancement of the cystic components (arrows).
Following radiological diagnosis, initial craniotomy and resection were performed (October 2008), with gross total resection (GTR) being satisfactorily achieved. Indeed, the postoperative MRI demonstrated postsurgical gliotic changes with focal atrophy in the left temporal lobe with no evidence of residual tumor [
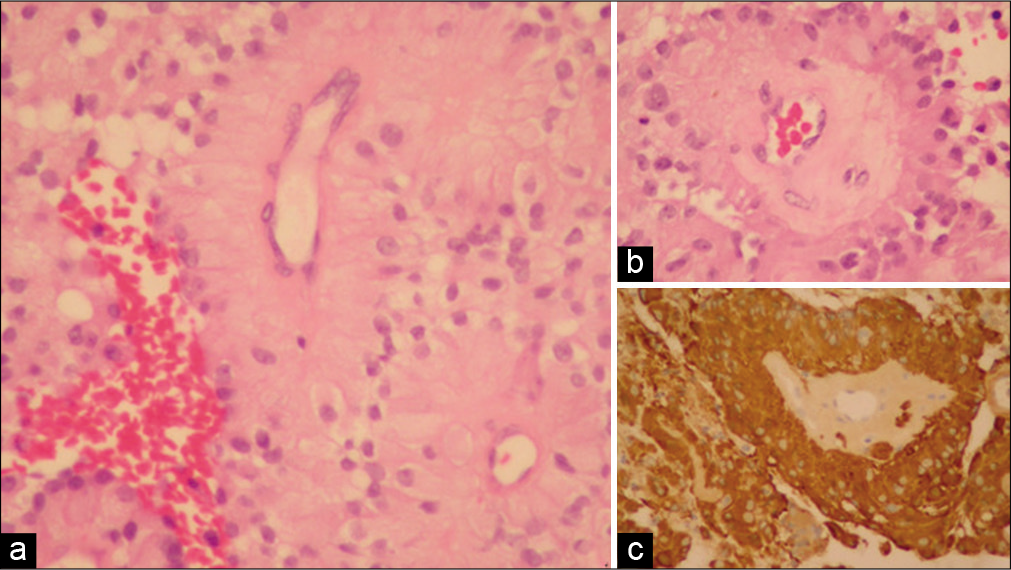
Figure 3:
A set of light microscopy images from tissue samples taken in October 2008 at initial surgery demonstrating (a) perivascular rosettes of tumor cells with broad processes passing down to the outside of central small blood vessels, (b) hyalinization of the blood vessel wall, and (c) positive (brown) immunostaining for glial fibrillary acid protein.
Initial postoperative management consisted of close surveillance with 3 monthly MR imaging, with clinical follow-up thereafter. At initial follow-up in May 2009, the patient was clinically well with the MRI demonstrating postoperative changes only with no visible residual or recurrent tumor.
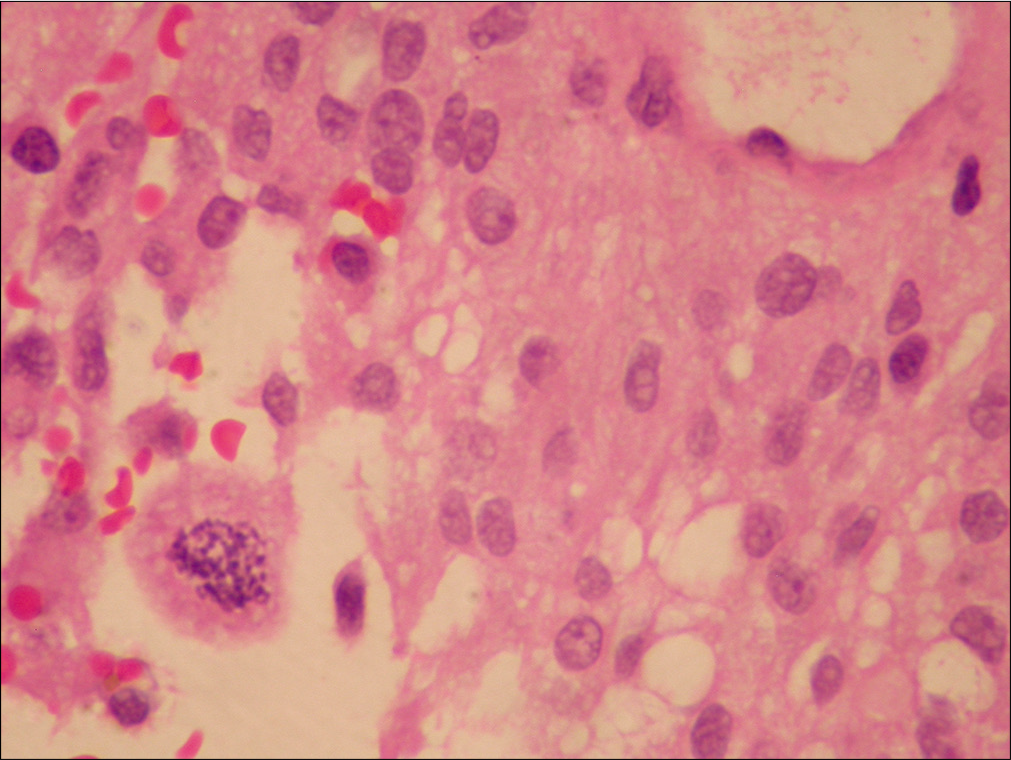
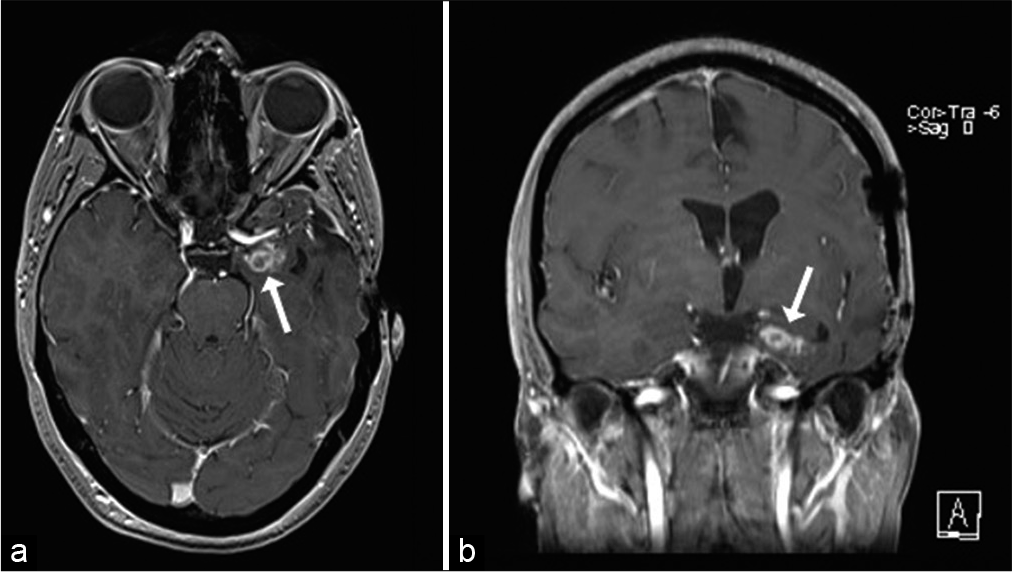
At 26-month postdiagnosis (December 2010), the corresponding MRI revealed two lesions in the left temporal surgical site. One anteriorly, in close proximity to the optic nerve, with the second more superior and posterior, located within the Sylvian fissure. At this stage, re-do craniotomy with excision of the recurrent tumor was decided on. Ultimately, GTR was again achieved, with histological analysis revealing frequent mitoses and more cytological atypia, suggesting the presence of a high-grade astroblastoma.
Adjunct treatment was discussed at this stage; considering the rapid timeline of recurrence and potential high-grade transformation, “glioblastoma-targeted” treatment was offered. The patient underwent concurrent radical chemoradiotherapy (60 Gy in 30 fractions along with 75 mg/m2 temozolomide daily) given from February to March 2011. On completion, she was started on cyclic single-agent temozolomide (cycle 1: 150 mg/m2 daily for 5 days; cycles 2–6: 200 mg/m2 daily for 5 days). Unfortunately, due to excessive suppression of the bone marrow despite of subsequent dose reduction, cyclic chemotherapy had to be discontinued after just two cycles (May–June 2011).
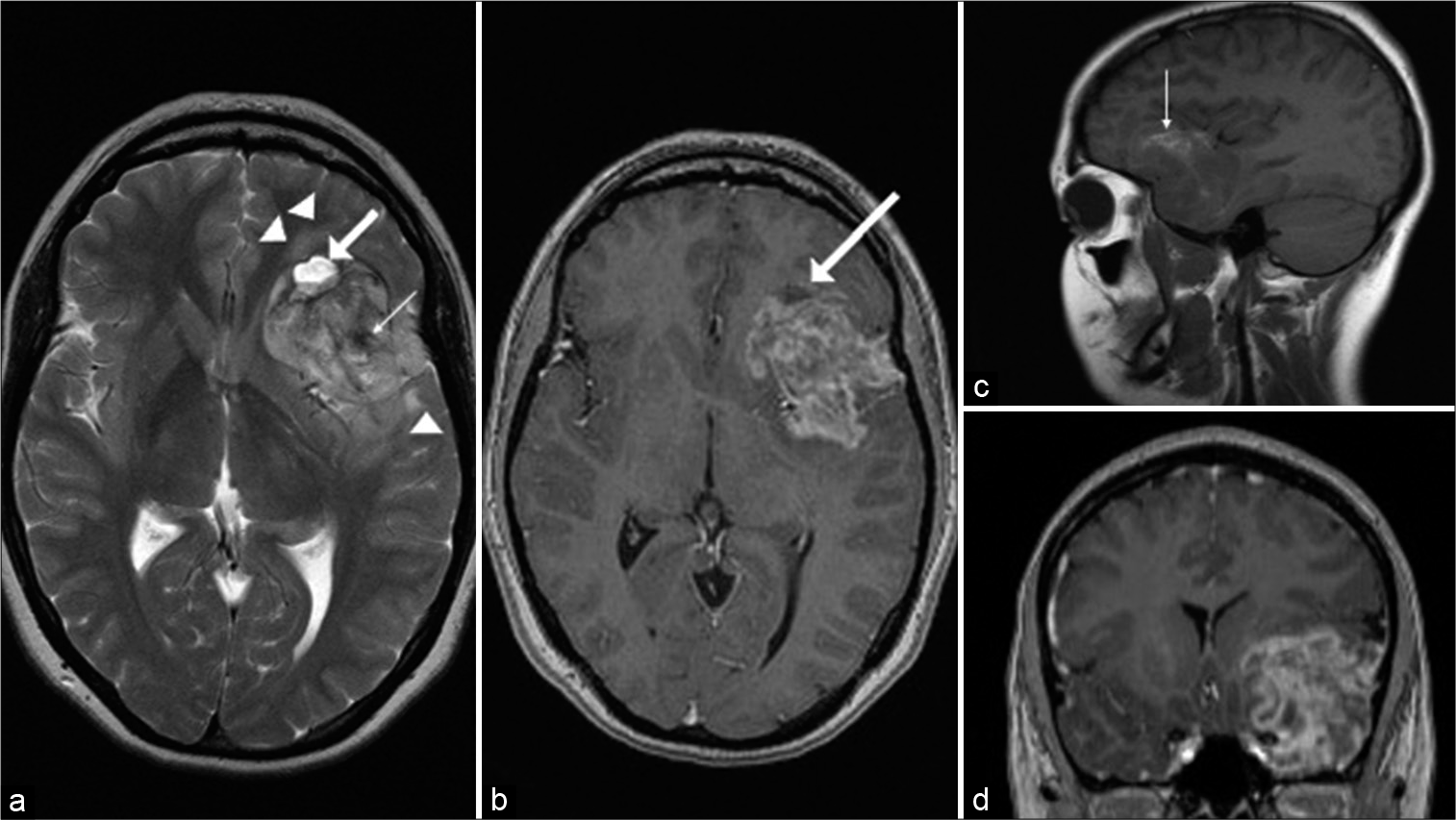
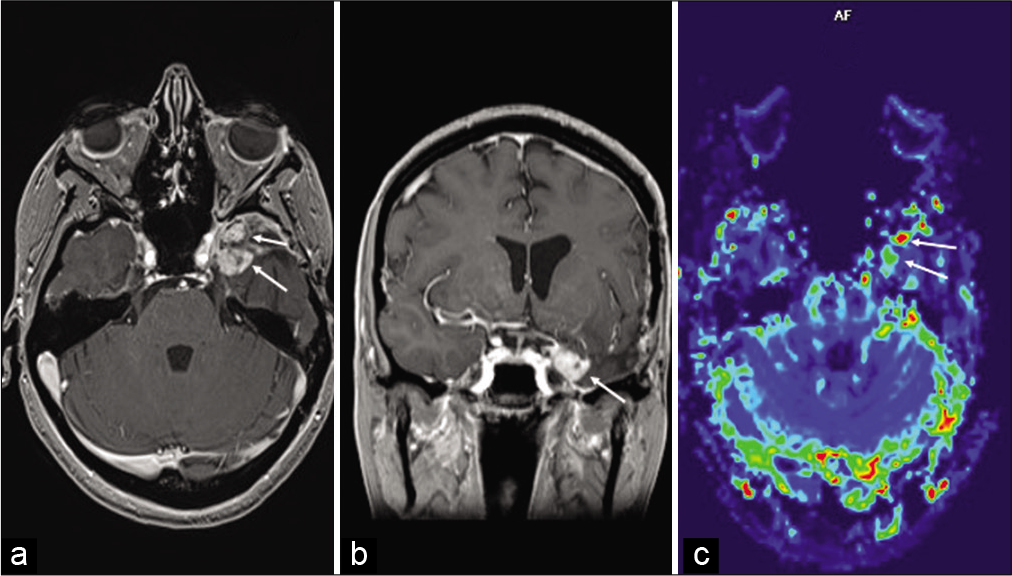
During routine MR surveillance in January 2014, an asymptomatic relapse was identified, and the patient was treated with Gamma Knife radiosurgery. Despite of the latter, the tumor recurred in August 2015 and the patient was reoperated with craniotomy and excision; the pathology reports confirmed the recurrence, with tumor cells present in the scar tissue at the site of the previous Gamma Knife surgery. This process of recurrence occurred again in September 2016 [
Figure 5:
(July 2020): axial (a) and coronal (b) postcontrast T1-weighted images showing recurrent nodular enhancing tumor (arrows) along the inferior aspect of the medial temporal lobe, also showing increased perfusion on the relative cerebral blood flow map (c) obtained from dynamic susceptibility contrast planar imaging.
Despite the recurrences, transformation, and progression of this high-grade histology, the patient remained clinically stable at the time of paper submission, with a performance status of 0 and no new or evolving neurological symptoms. Further chemotherapy with reduced temozolomide will be considered in case of recurrence on upcoming interval imaging.
DISCUSSION
General aspects
Astroblastomas are infrequent neoplasms of the CNS, accounting for approximately 0.48–2.80% of all gliomas.[
On MRI, astroblastomas are typically seen as large, lobulated, supratentorial, well-demarcated, solid, and cystic masses.[
Treatment: trends and controversies
Ahmed et al. presented the largest series of astroblastoma cases in 2013 (n = 239), followed by a subsequent review of 54 cases by Hammas et al. in 2018.[
Similarly, Hammas et al. commented on the benefits of GTR over subtotal resection, stating the benefits of the former in providing superior tumor control rates. Also mentioned was that the addition of adjuvant focal radiotherapy after subtotal resection does not appear to provide equivalent outcomes to GTR. Also, the authors reiterated the overall benefits of adjuvant therapy for high-grade and recurrent cases.[
In terms of recurrence, the role of chemotherapy remains a question of debate; despite the controversy, some groups have reported on the benefit of systemic treatment in some of these patients.[
Based on our institutional experience and previous review of the medical literature, we strongly recommend the use of temozolomide in cases of high-grade histology recurring at postsurgery and/or postradiation; as it was the case here, a satisfactory degree of response might be achieved, even at reduced doses. Should temozolomide not be indicated due to local failure or toxicity, PCV (alternatively PC or single-agent CCNU depending on the patient’s fitness to chemotherapy) might prove beneficial in terms local control although bone marrow toxicity remains a precluding factor, particularly at full dose.
Histogenesis and molecular profile: A complex subject
Despite being part of the diagnostic neuro-oncology portfolio since 1926, the histogenesis of astroblastoma continues to be debated by neuropathologists. This comes as no surprise as these glial neoplasms contain complex features overlapping both astrocytomas and ependymomas as well as other entities such as high-grade neuroepithelial tumor with MN1 alteration (HGNET-MN1).[
In the case presented here, molecular profiling was not carried out at initial diagnosis as molecular profiling was not available then. Despite this, the tumor was diagnosed as an astroblastoma according to the current WHO classification, that is, based on the histological features of the neoplasm. As a result, some molecular analyses were subsequently carried out on the recurrent tumor (2010). A nonsense variant in RB1 and a frameshift variant in PTCH1 were identified while no pathogenic variants were detected in BRAF. Moreover, no mutations were detected in the following gene panel: ALK, ATRX, BCOR, CDKN2A, CDKN2B, CTNNB1, DDX3X, EZH2, FGFR1, H3F3A, HIST1H3B, HIST1H3C, IDH1, IDH2, MSH6, MYCN, NF1, NF2, PMS2, PTEN, SMARCA4, SMARCB1, SMO, SUFU, TERT, TP53, TSC1, TSC2, and YAP1 genes. HIST2H3C analysis failed. Of note again, although MN1 testing was not available at that particular point in time, the above collected data excluded the presence of CNS primaries other than astroblastoma.[
High-grade transformation capability and its impact on survival
It is important to note that astroblastomas have not yet been given a WHO classification grade and that the histopathologic subtyping into low and high grade, although used locally by some centers, has not yet been fully integrated in the formal WHO classification, which makes the literature somehow inconsistent. However, we believe that subtyping remains a crucial element of diagnosis likely to shape management.
Indeed, as early as 1989, Bonnin and Rubinstein categorized astroblastoma into low- and high-grade subtypes, with high-grade tumors demonstrating particular features such as microvascular proliferation, necrosis with pseudopalisades, increased cellularity, and nuclear atypia with a high mitotic MIB-1 proliferative index.[ In the case of low-grade tumors, complete resection may indeed be curative and appears to play a major role in postsurgery outcome for both grades of astroblastoma.[ In accordance with our review of the literature, adjuvant treatment appears to remain critical in the management of high-grade lesions. In this patient case, radiotherapy and radiosurgery might have deferred recurrence to some degree, at least in theory. In contrast, the tumor seemed to be highly sensitive to temozolomide and PCV chemotherapy, even on a reduced dose schedule. In spite of the scarce clinical evidence and the ensuing lack of standard protocols for patients presenting with astroblastoma,[
CONCLUSION
We present a rare case of high-grade astroblastoma with complex evolution. To the best of our knowledge, this is the longest surviving case anaplastic astroblastoma in the available medical literature. Alongside increasing numbers of reported cases of astroblastoma, emerging histomolecular analysis of these tumors will further enhance clinical decisions surrounding the management of astroblastomas. In the majority of cases, management requires customized intervention, including surgery, radiotherapy, and chemotherapy. However, the rarity of the entity makes prospective studies hardly feasible and treatment protocols difficult to achieve. In this context, closer international cooperation surrogate to specific databases and multidisciplinary task groups are warranted and encouraged.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal V, Mally R, Palande DA, Velho V. Cerebral astroblastoma: A case report and review of literature. Asian J Neurosurg. 2012. 7: 98-100
2. Ahmed KA, Allen PK, Mahajan A, Brown PD, Ghia AJ. Astroblastomas: A Surveillance, Epidemiology, and End Results (SEER)-based patterns of care analysis. World Neurosurg. 2014. 82: e291-7
3. Alaraj A, Chan M, Oh S, Michals E, Valyi-Nagy T, Hersonsky T. Astroblastoma presenting with intracerebral hemorrhage misdiagnosed as dural arteriovenous fistula: Review of a rare entity. Surg Neurol. 2007. 67: 308-13
4. Bailey P, Bucy PC. Astroblastoma of the brain. Acta Psychiatr Neurol. 1930. 5: 308-13
5. Bailey P, Cushing HA. A classification of tumors of the glioma group on a histogenetic basis with a correlation study of prognosis. Lippincott. 1926. 83-84: 133-6
6. Bell JW, Osborn AG, Salzman KL, Blaser SI, Jones BV, Chin SS. Neuroradiologic characteristics of astroblastoma. Neuroradiology. 2007. 49: 203-9
7. Bergkåsa M, Sundstrøm S, Gulati S, Torp SH. Astroblastoma a case report of a rare neuroepithelial tumor with complete remission after chemotherapy. Clin Neuropathol. 2011. 30: 301-6
8. Bhalerao S, Nagarkar R, Adhav A. A case report of high-grade astroblastoma in a young adult. CNS Oncol. 2019. 8: CNS29
9. Bonnin JM, Rubinstein LJ. Astroblastomas: A pathological study of 23 tumors, with a postoperative follow-up in 13 patients. Neurosurgery. 1989. 25: 6-13
10. Chen W, Soon YY, Pratiseyo PD, Sutanto R, Hendriansyah L, Kuick CH. Central nervous system neuroepithelial tumors with MN1-alteration: An individual patient data meta-analysis of 73 cases. Brain Tumor Pathol. 2020. 37: 145-53
11. Cunningham DA, Lowe LH, Shao L, Acosta NR. Neuroradiologic characteristics of astroblastoma and systematic review of the literature: 2 new cases and 125 cases reported in 59 publications. Pediatr Radiol. 2016. 46: 1301-8
12. D’Cruze L, Sundaram S, Iyer S, Ganesh K. A rare case of a high-grade astroblastoma with 5-year follow-up. Asian J Neurosurg. 2021. 16: 183-6
13. Hammas N, Senhaji N, Alaoui Lamrani MY, Bennis S, Chaoui EM, El Fatemi H. Astroblastoma a rare and challenging tumor: A case report and review of the literature. J Med Case Rep. 2018. 12: 102
14. Kim DS, Park SY, Lee SP. Astroblastoma: A case report. J Korean Med Sci. 2004. 19: 772-6
15. Kristensen BW, Priesterbach-Ackley LP, Petersen JK, Wesseling P. Molecular pathology of tumors of the central nervous system. Ann Oncol. 2019. 30: 1265-78
16. Lehman NL, Hattab EM, Mobley BC, Usubalieva A, Schniederjan MJ, McLendon RE. Morphological and molecular features of astroblastoma, including BRAFV600E mutations, suggest an ontological relationship to other cortical-based gliomas of children and young adults. Neuro Oncol. 2017. 19: 31-42
17. Lehman NL, Usubalieva A, Lin T, Allen SJ, Tran QT, Mobley BC. Genomic analysis demonstrates that histologically-defined astroblastomas are molecularly heterogeneous and that tumors with MN1 rearrangement exhibit the most favorable prognosis. Acta Neuropathol Commun. 2019. 7: 42
18. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
19. Mellai M, Piazzi A, Casalone C, Grifoni S, Melcarne A, Annovazzi L. Astroblastoma: Beside being a tumor entity, an occasional phenotype of astrocytic gliomas?. Onco Targets Ther. 2015. 8: 451-60
20. Merfeld EC, Dahiya S, Perkins SM. Patterns of care and treatment outcomes of patients with astroblastoma: A national cancer database analysis. CNS Oncol. 2018. 7: CNS13
21. Mhatre R, Sugur HS, Nandeesh BN, Chickabasaviah Y, Saini J, Santosh V. MN1 rearrangement in astroblastoma: Study of eight cases and review of literature. Brain Tumor Pathol. 2019. 36: 112-20
22. Navarro R, Reitman AJ, de León GA, Goldman S, Marymont M, Tomita T. Astroblastoma in childhood: Pathological and clinical analysis. Childs Nerv Syst. 2005. 21: 211-20
23. Port JD, Brat DJ, Burger PC, Pomper MG. Astroblastoma: Radiologic-pathologic correlation and distinction from ependymoma. AJNR Am J Neuroradiol. 2002. 23: 243-7
24. Shen F, Chen LC, Yao Y, Zhou LF. Astroblastoma: Rare incidence and challenges in the pattern of care. World Neurosurg. 2014. 82: e125-7
25. Singh DK, Singh N, Singh R, Husain N. Cerebral astroblastoma: A radiopathological diagnosis. J Pediatr Neurosci. 2014. 9: 45-7
26. Sughrue ME, Choi J, Rutkowski MJ, Aranda D, Kane AJ, Barani IJ. Clinical features and post-surgical outcome of patients with astroblastoma. J Clin Neurosci. 2011. 18: 750-4
27. Ujihara M, Mishima K, Sasaki A, Adach JI, Shirahata M, Suzuki T. Unique pathological findings of astroblastoma with MN1 alteration in a patient with late recurrence. Brain Tumor Pathol. 2021. 38: 243-9
28. Wood MD, Tihan T, Perry A, Chacko G, Turner C, Pu C. Multimodal molecular analysis of astroblastoma enables reclassification of most cases into more specific molecular entities. Brain Pathol. 2018. 28: 192-202