- Department of Neurosurgery, Coimbra University Hospital Centre, Coimbra, Portugal.
- Faculty of Medicine, University of Coimbra, Coimbra, Portugal.
DOI:10.25259/SNI_856_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniela Matos1,2, Ricardo Pereira1,2. Thoracic spine schwannoma presenting with traumatic spinal cord injury: A case report. 31-May-2021;12:251
How to cite this URL: Daniela Matos1,2, Ricardo Pereira1,2. Thoracic spine schwannoma presenting with traumatic spinal cord injury: A case report. 31-May-2021;12:251. Available from: https://surgicalneurologyint.com/surgicalint-articles/10832/
Abstract
Background: The presentation of a thoracic spinal tumor due to high-impact trauma is quite rare and we found no other case reported.
Case Description: This is a case report and literature review. A patient presented with severe paraparesis on day 4 after trauma. Thoracic MRI showed an oval image centered to T4-T5 suggestive of hemorrhage. The patient underwent a bilateral T4 and T5 laminectomy and microsurgically assisted intradural exploration. After laminectomy, we found no extradural lesions, so we proceeded to dural opening, after which we found a large extramedullary lesion which was completely removed. Pathology revealed a schwannoma. The patient had a very good recovery after surgery and motor rehabilitation. At 6 months after surgery, inferior limbs muscle strength was completely normal. We found no other case reported.
Conclusion: Thoracic spine schwannomas are difficult to early diagnose unless there is a clinical suspicion. Initial presentation as bleeding after trauma was not described before. This presentation should be kept in the differential diagnosis of any patient with an acute neurological deficit without trauma signs on admission imaging.
Keywords: Spine cord injury, Spine trauma, Spine tumor, Thoracic spine schwannoma, Tumor bleeding
INTRODUCTION
Spinal schwannomas are the most common intradural extramedullary spinal tumors, representing up to one-third of these lesions.[
The following is a rare case of an intradural nerve sheath schwannoma presenting with motor weakness after trauma.
CLINICAL PRESENTATION
A young male patient, with no significant previous medical history, was admitted to the emergency room after a 6 m high roof fall, with right-sided limb trauma and left inferior limb motor weakness. The patient had acute right inferior limb pain and was unable to walk unaided. Neurological examination disclosed mild (Grade 4/5) weakness of the left thigh extension and a very painful active and passive mobilization of the right-sided limbs. He also had bilateral inferior limb numbness, American Spinal Injury Association Score D.
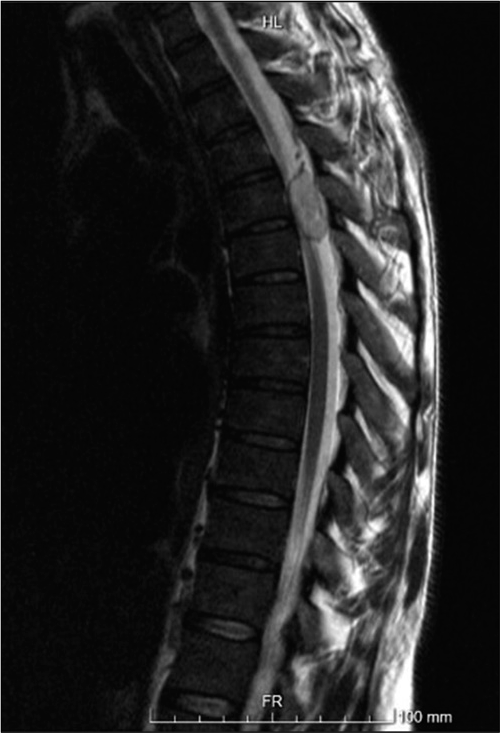
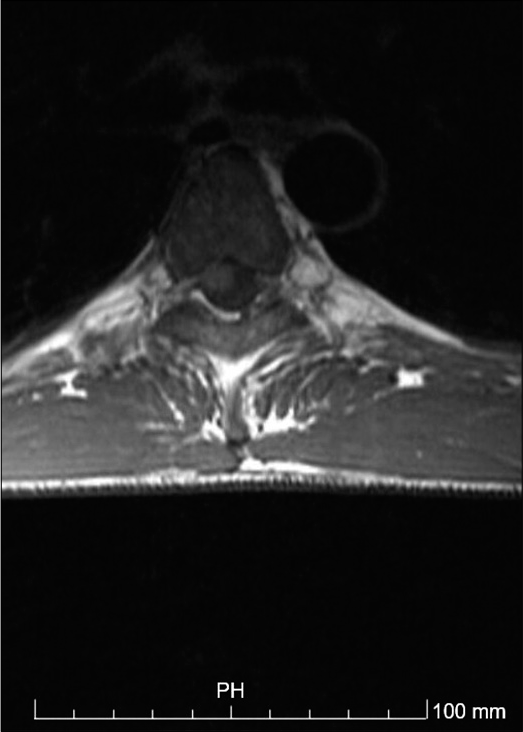
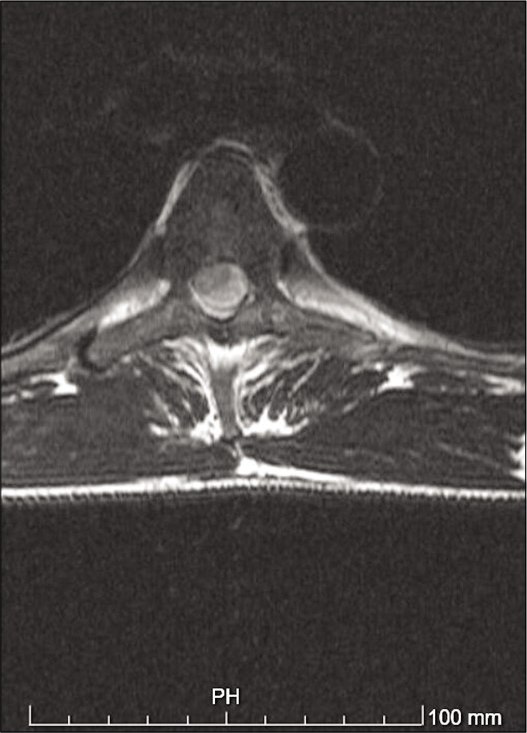
A thoracolumbar spine CT scan was performed and showed no traumatic lesions. A trochanteric right femur and right olecranon fractures were diagnosed and treated. The patient was then admitted to the orthopedics unit. At day 4 after trauma, the patient developed severe paraparesis (Grade 1/5), American Spinal Injury Association Score C, with D9 sensory level and urinary retention. Dorsal and lumbar spine magnetic resonance was immediately performed and showed an oval shaped image centered to D4-D5, compressing the spinal cord, with surrounding edema [
Operative findings
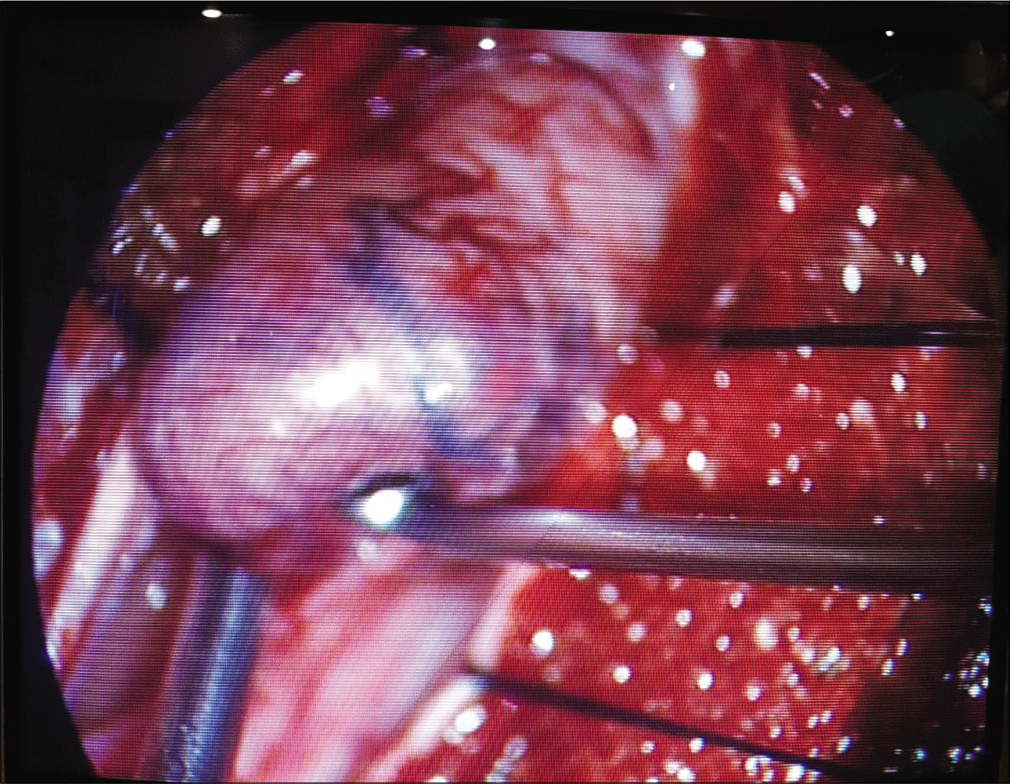
The patient underwent T4-T5 bilateral laminectomy under radiological control. We found no extradural lesions and so we proceeded to dural opening. As soon as the duramater was fully opened, we found a large reddish-grayish firm mass with approximately 2 cm in diameter, with a thin surrounding blood clot. The tumor, which had clear limits, was carefully dissected [
Histology
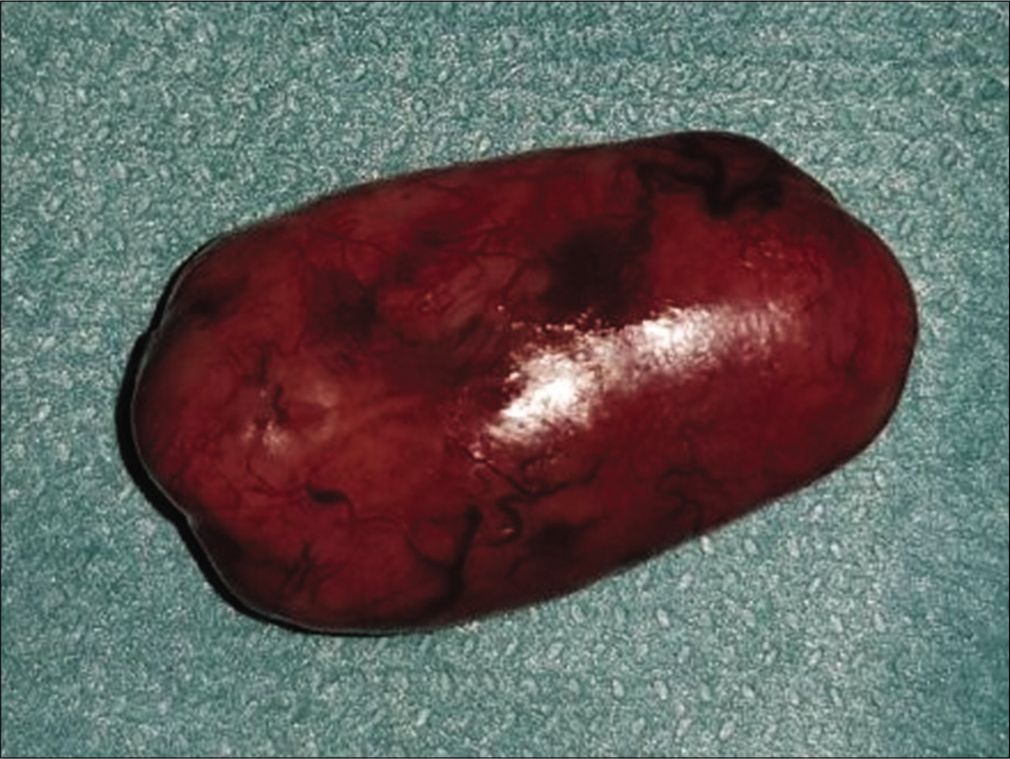
Received in formalin, the surgical piece consisted of a grayish, nodular lesion with 2.8 × 1.5 × 1.6 cm in diameters [
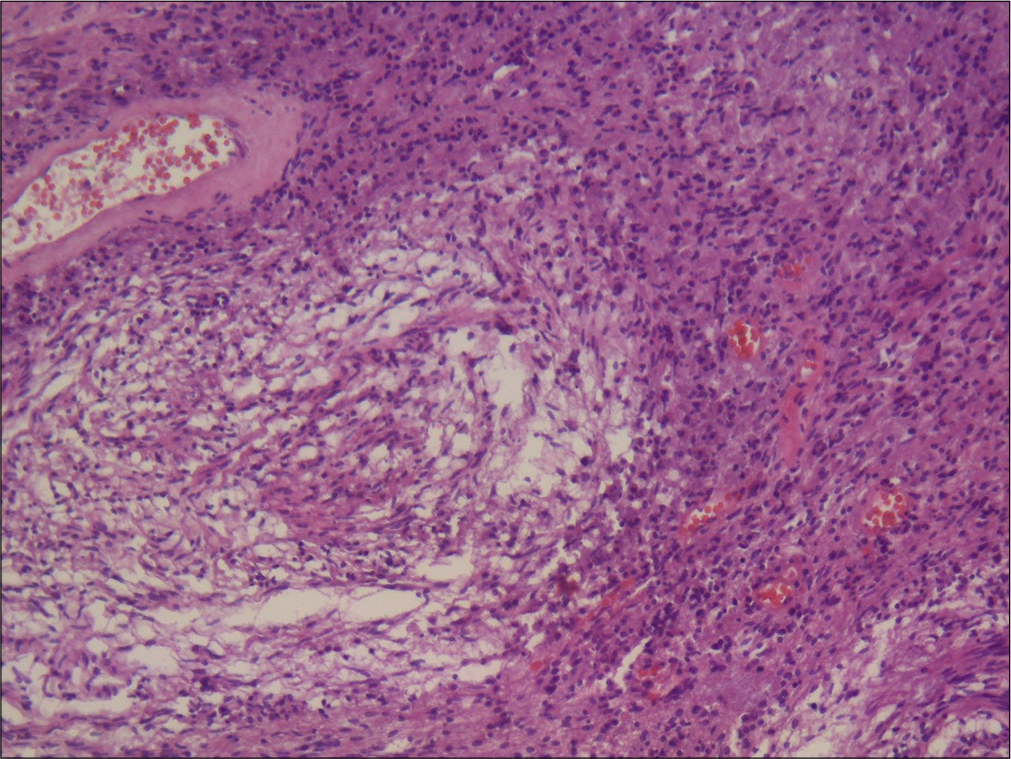
Histology revealed a benign nerve sheath tumor surrounded by a thin capsule of dense connective tissue with a variable cellular density. It showed predominant areas of elongated eosinophilic cytoplasm cells originating parallel or crossed bundles (Antoni A type areas) with some minor less cellular areas of loose tissue and microcysts. These cells had rounded nuclei and undefined cytoplasm (Antoni B type areas). The tumor vessels had thick and hyalinized walls and, in some areas, these vessels were agglomerated and dilated with an angiomatous aspect. There were focal intra and extracellular deposits of hemosiderin with areas of recent hemorrhage. The tumor was diffusely positive for S100 protein.
Postoperative outcomes
The patient showed neurological improvement immediately after surgery, with better mobilization of the left inferior limb (Grade 3/5). The right inferior limb was still limited by the trochanteric fracture, but also showed increased muscular strength in the right foot (Grade 4/5). Urinary sphincter dysfunction resolved within 1 month.
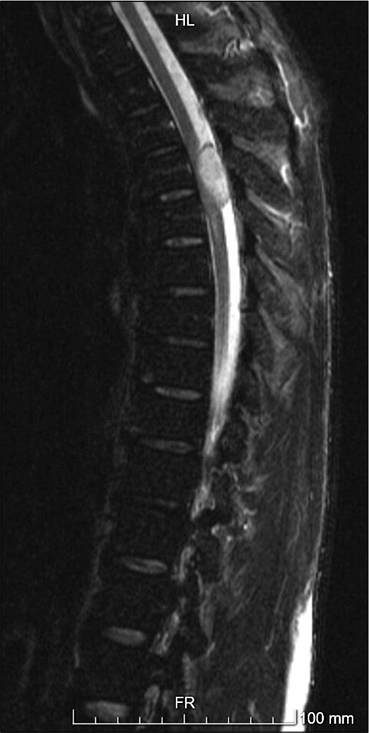
A thoracic spine MRI postsurgery showed total lesion removal and did not demonstrate any other lesions. Two months later, the patient could walk unassisted. On further follow-up, the patient totally recovered muscular strength, ASIA score E. At 1.5 years follow–up, the patient remains with American Spinal Injury Association Score E.
DISCUSSION
To the best of our knowledge, this is the first reported case of thoracic spinal schwannoma presenting days after high-impact trauma.[
In hindsight, regarding the left inferior limb motor weakness, the patient developed after trauma, a thoracolumbar MRI should have been done earlier, as the initial thoracolumbar CT showed no signs of spinal trauma and the neurological deficit was already present. Probably, the initial spinal cord insult and the following increasing edema were the cause of this paraparesis and subsequent neurological worsening. Once established, decompression was needed, and it was achieved by tumor removal. This procedure and a short period of anti-edema therapy resulted in a very good outcome.
The blood deposits found in the conus medullaris area and inside the tumor could have been explained by the trauma insult to the tumor itself causing its vessels to rupture and bleed. An alternative explanation is the stress that this patient was submitted to, not only by the trauma but also by the following hospitalization and treatments, could have sensitized the tumor and causing it to bleed the following days after the trauma, which would also justify the surrounding edema and the absence of bleeding signals in the initial CT scan.
Probably, the high-impact trauma caused spinal cord and tumoral insults, both resulting in tumor bleeding and surrounding edema leading to paraparesis.[
CONCLUSION
Thoracic spine schwannomas, as other preexisting space-occupying lesions, should be kept in mind as a differential diagnosis of acute spinal trauma patients with paraparesis and without bony traumatic lesions. Although the underlying mechanism may not be clear, we report a case of a previously healthy patient with severe paraparesis after trauma in relation with a thoracic spine schwannoma with hemorrhage. After surgery, this patient had a great recovery having normal inferior limbs muscular strength after 2 months.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Brown K, Dean A, Sharr M. Thoracic intramedullary schwannoma. Neuropathol Appl Neurobiol. 2002. 28: 421-4
2. Cohen ZR, Knoller N, Hadani M, Davidson B, Nass D, Ram Z. Traumatic intratumoral hemorrhage as the presenting symptom of a spinal neurinoma. Case report. J Neurosurg. 2000. 93: 327-9
3. Ge L, Arul K, Ikpeze T, Baldwin A, Nickels JL, Mesfin A. Traumatic and nontraumatic spinal cord injuries. World Neurosurg. 2018. 111: e142-8
4. Ge L, Arul K, Mesfin A. Spinal cord injury from spinal tumors: Prevalence, management, and outcomes. World Neurosurg. 2019. 122: e1551-6
5. Hdeib A, Goodwin CR, Sciubba D, Bydon A, Wolinsky JP, Witham T. Hemorrhagic thoracic schwannoma presenting with intradural hematoma and acute paraplegia after spinal manipulation therapy. Int J Spine Surg. 2016. 10: 42
6. Jenkins AL, Ahuja A, Oliff AH, Sobotka S. Spinal Schwannoma presenting due to torsion and hemorrhage: Case report and review of literature. Spine J. 2015. 15: e1-4
7. Koeller KK, Shih RY. Intradural extramedullary spinal neoplasms: Radiologic-pathologic correlation. Radiographics. 2019. 39: 468-90
8. Mehta AI, Adogwa O, Karikari IO, Thompson P, Verla T, Null UT. Anatomical location dictating major surgical complications for intradural extramedullary spinal tumors: A 10-year single-institutional experience. J Neurosurg Spine. 2013. 19: 701-7
9. Ottenhausen M, Ntoulias G, Bodhinayake I, Ruppert FH, Schreiber S, Förschler A. Intradural spinal tumors in adults-update on management and outcome. Neurosurg Rev. 2019. 42: 371-88
10. Tanaka H, Kondo E, Kawato H, Kikukawa T, Ishihara A, Toyoda N. Spinal intradural hemorrhage due to a neurinoma in an early puerperal woman. Clin Neurol Neurosurg. 2002. 104: 303-5
11. Thiagarajan A, Yamada Y.editors. Primary spine tumors. Target Volume Delineation and Treatment Planning for Particle Therapy. Berlin: Springer; 2018. p.
12. Turel MK, D’Souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: An analysis of 164 patients. Neurosurg Focus. 2015. 39: E9
13. Xiao R, Miller JA, Abdullah KG, Lubelski D, Mroz TE, Benzel EC. Quality of life outcomes following resection of adult intramedullary spinal cord tumors. Neurosurgery. 2016. 78: 821-8