- Department of Neurosurgery, Nara Medical University, Kashihara, Japan.
DOI:10.25259/SNI_349_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takanori Furuta, Ichiro Nakagawa, HunSoo Park, Kenta Nakase, Shohei Yokoyama, Masashi Kotsugoi, Yasuhiro Takeshima, Hiroyuki Nakase. Thoracolumbar intraosseous spinal epidural arteriovenous fistulas after vertebral compression fracture: A case report and literature review. 07-Jun-2021;12:270
How to cite this URL: Takanori Furuta, Ichiro Nakagawa, HunSoo Park, Kenta Nakase, Shohei Yokoyama, Masashi Kotsugoi, Yasuhiro Takeshima, Hiroyuki Nakase. Thoracolumbar intraosseous spinal epidural arteriovenous fistulas after vertebral compression fracture: A case report and literature review. 07-Jun-2021;12:270. Available from: https://surgicalneurologyint.com/surgicalint-articles/10856/
Abstract
Background: The pathophysiology of spinal epidural arteriovenous fistulas (SEAVFs) with perimedullary venous drainage remains to be elucidated. This report describes a case of intraosseous SEAVF in a patient with a history of a thoracolumbar vertebral fracture at the same level 10 years before presenting with progressive myelopathy secondary to retrograde venous reflux into the perimedullary vein.
Case Description: A 71-year-old man presenting with progressive paraparesis was diagnosed with a SEAVF involving a previous Th12 and L1 vertebral compression fracture on which feeders from multiple segmental arteries converged. The interesting feature of this case was that the fistula was located in the fractured vertebral body. The fistula was totally obliterated by transarterial embolization of the segmental arteries followed by symptom improvement.
Conclusion: We presented a rare case of an intraosseous SEAVF secondary to a thoracolumbar compression fracture with perimedullary venous reflux causing progressive myelopathy. The fistula was located in the fractured vertebral body.
Keywords: Intraosseous epidural arteriovenous fistulas, Trans-arterial embolization, Vertebral compression fracture
INTRODUCTION
Spinal epidural arteriovenous fistulas (SEAVFs), in particular intraosseous SEAVFs, are rare among spinal vascular lesions.[
CASE REPORT
A 71-year-old man presented with a 2-month history of gradually progressive paraparesis. In addition, he also had a 10-year history of a fractured Th12 and L1 vertebral body due to a fall. His symptoms had gradually progressed to the point where he had bladder dysfunction and difficulty walking. On magnetic resonance (MR) imaging [
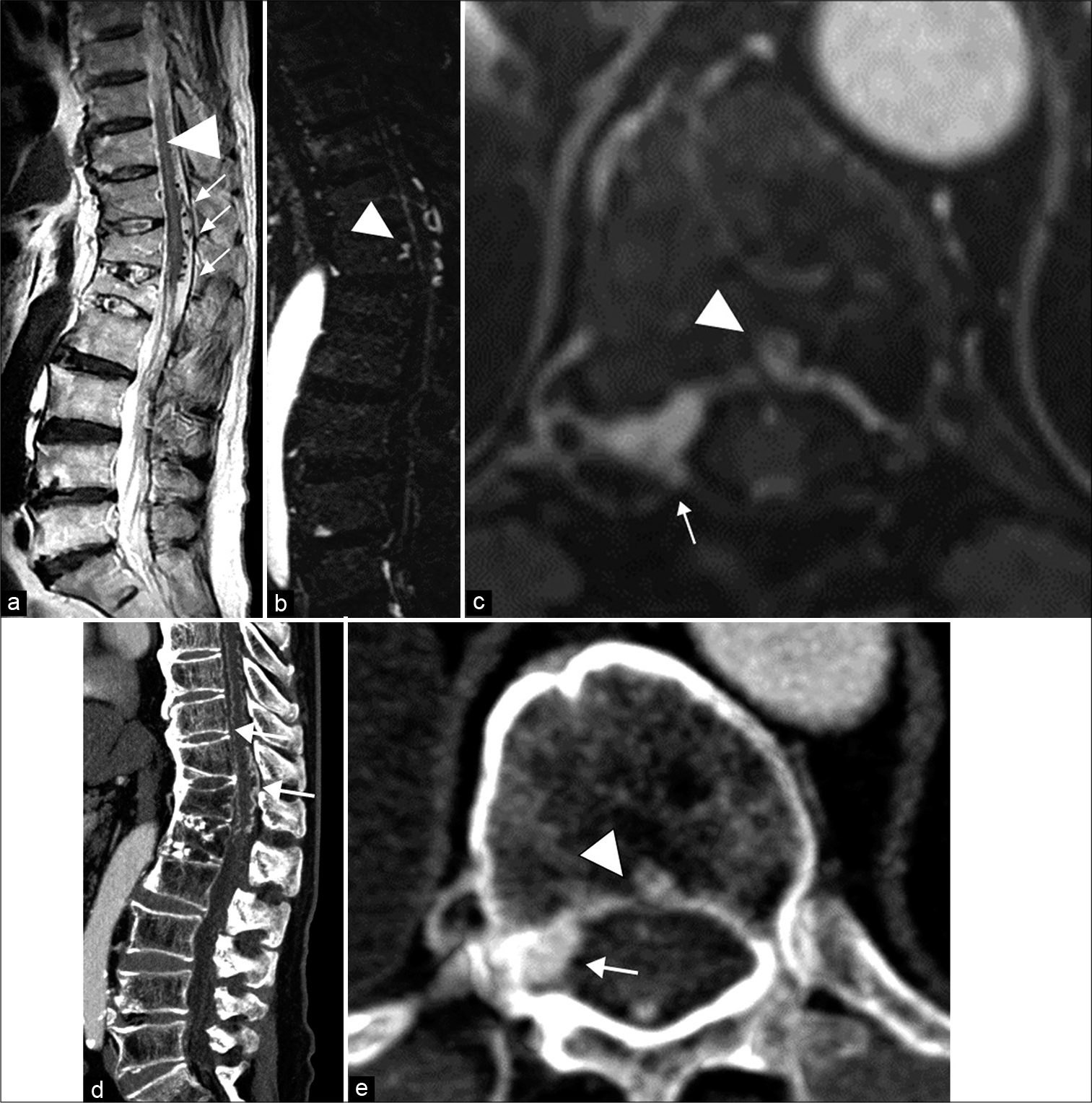
Figure 1:
(a) Sagittal T2-weighted MR image shows high signal intensity and edematous changes in the spinal cord (arrowhead). Enlarged perimedullary veins are depicted anterior and posterior to the spinal cord (arrows). (b) Sagittal enhanced T1-weighted MR image shows an intraosseous fistula in the Th12 vertebral body. (c) Axial T1-weighted Gd-enhanced VIBE MR image shows a hyperenhanced cavity (arrowhead) in the Th12 vertebral body and the epidural venous pouch (arrow). (d) Sagittal enhanced CT scans show the previous Th12 and L1 compression fracture, and longitudinally enlarged perimedullary veins. (e) Axial enhanced CT scans show a hyperenhanced cavity (arrowhead) in the Th12 vertebral body and the epidural venous pouch (arrow).
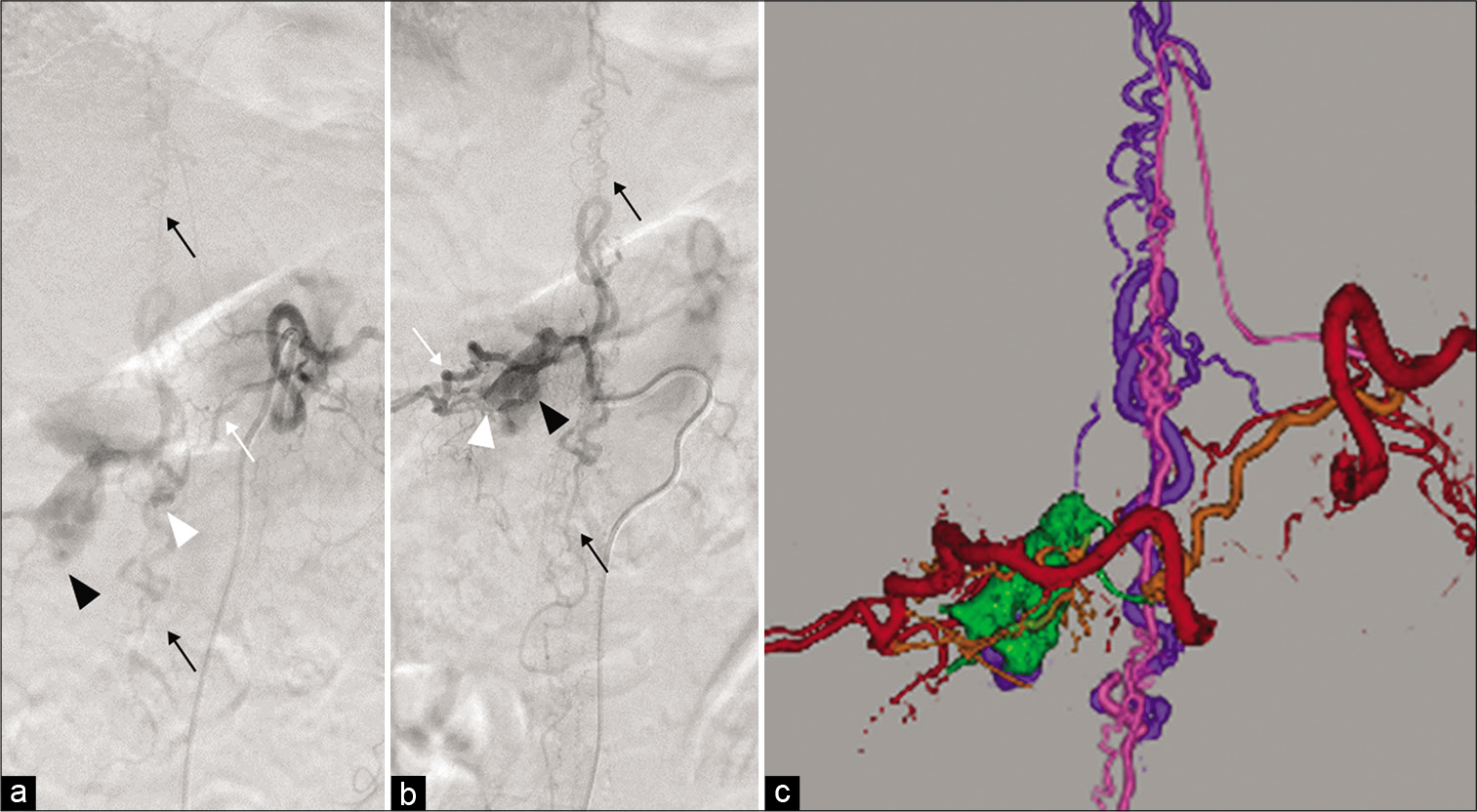
Figure 2:
Selective spinal angiography of the dorsal somatic branch (white arrow) of the left Th11 (a) and the right Th12 (b) segmental artery reveals an epidural arteriovenous fistula (white arrowhead) with an epidural venous pouch (black arrowhead) draining through the intradural vein into the perimedullary vein (black arrows). (c) 3D fusion image merged with spinal angiography from the left Th11 and right Th12 segmental arteries shows the segmental artery (red), feeders (orange), epidural venous pouch (green), drainers (purple), and perimedullary vein (pink).
Treatment
We planned endovascular transarterial embolization to eliminate the fistula. The endovascular procedure was performed after obtaining written informed consent from the patient. Under local anesthesia, DeFrictor flow-directed microcatheters (MEDIVOCO’S HIRATA, Osaka, Japan) were introduced into the right dorsal somatic branch of the Th12 segmental artery as close as possible to the shunt points. The feeding arteries, venous pouch, and proximal portion of the draining veins were embolized with 12.5% heated glue (a mixture of n-butyl cyanoacrylate [NBCA] and lipiodol at a ratio of 1:7). Subsequently, transarterial embolization of the left dorsal somatic branch of the Th11 segmental artery was performed in the same manner. The final angiogram revealed complete obliteration of the fistula [
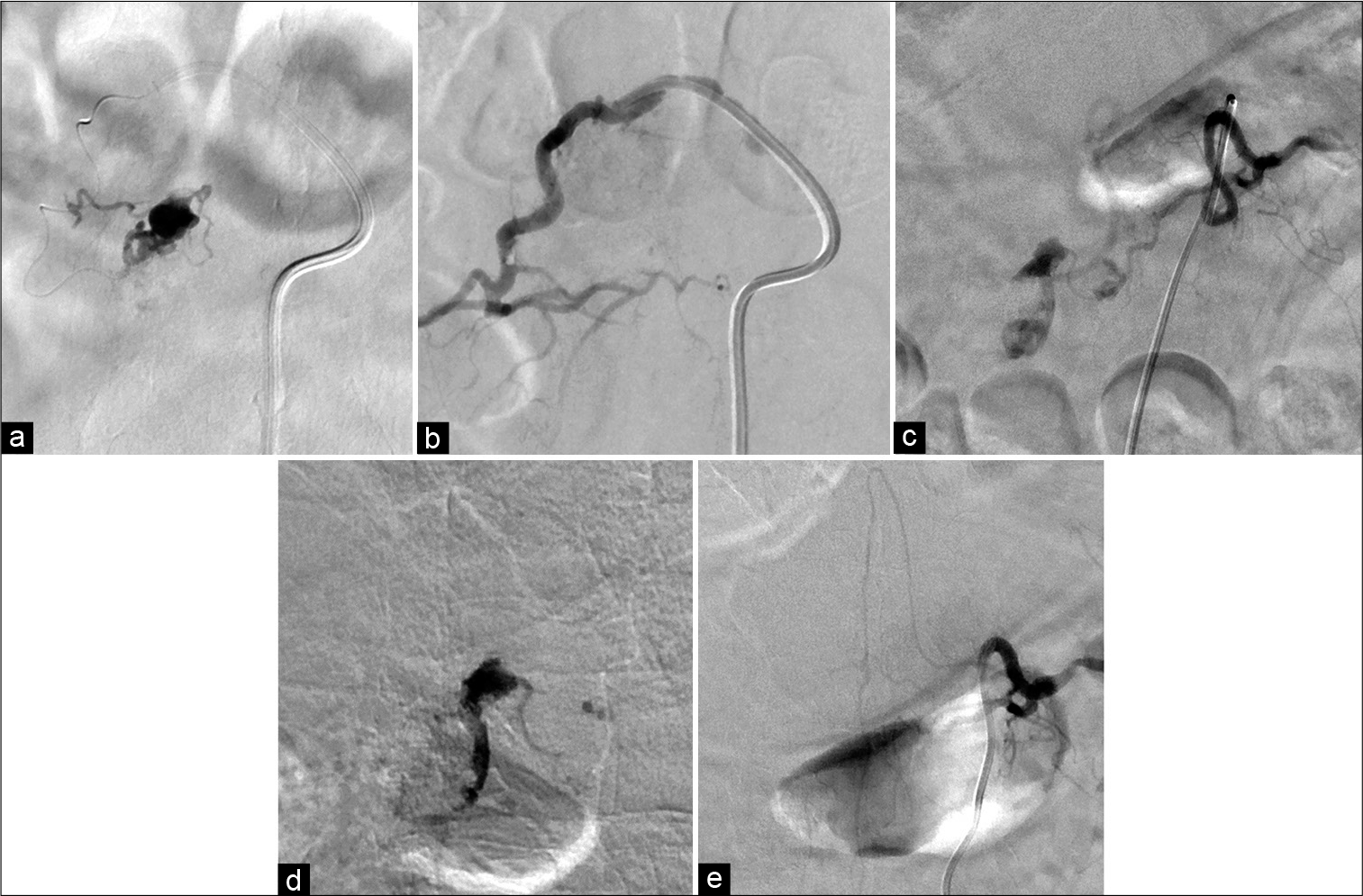
Figure 3:
Transarterial embolization with 12.5% heated NBCA was performed through the dorsal somatic branch of the right Th12 segmental artery (a), and complete obliteration of the fistula from the Th12 segmental artery was obtained (b). Subsequently, transarterial embolization with NBCA was performed through the dorsal somatic branch of the left Th11 segmental artery (c and d), and total obliteration of the fistula was obtained (e).
Postoperative course
The patient’s neurological symptoms including paresthesia of the lower extremities, gait disturbance, and urinary sphincter dysfunction improved after the treatment. Three-month follow-up MR imaging revealed disappearance of the spinal cord swelling and the signal voids in the vicinity of the spinal cord. No recurrence was observed 2 years after the treatment [
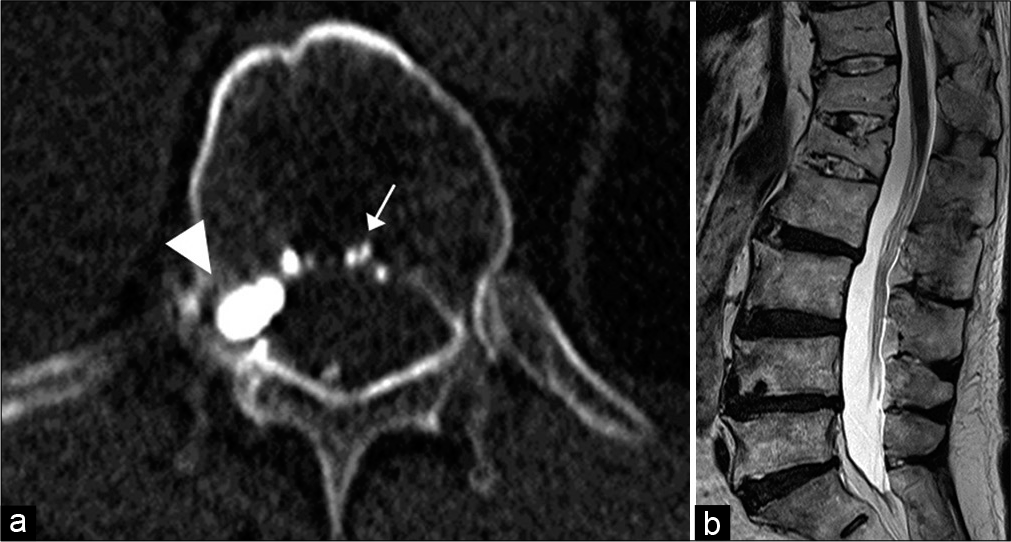
Figure 4:
CT (a) after embolization shows the glue cast in the intraosseous fistula (arrow) in the Th12 vertebral body and the ventral epidural pouch (arrowhead). Six-month follow-up sagittal T2-weighted MR image (b) of the thoracolumbar spine reveals the disappearance of the perimedullary venous structures and normal spinal cord signal intensity.
DISCUSSION
SEAVFs are rare among spinal vascular lesions. The precise mechanisms responsible for the development and growth of epidural AVFs remain unclear. However, some reports have suggested a relationship between these entities and trauma, prior surgery, or neurofibromatosis.[
This case has several interesting findings. The most peculiar finding was that the fistula was located in the same level of the fractured vertebral body. A fistula may form due to an increase in local venous pressure and inflammation after a vertebral body fracture, resulting in formation of a shunted epidural venous pouch. We speculate that the fistula in the fractured vertebral body was mainly fed from the left Th11 segmental artery and with development of the shunt to the epidural venous pouch. As a result, secondary fistula fed the right Th12 segmental artery into the epidural venous pouch, resulting in subsequent development of the epidural venous pouch. The SEAVF progressed further, and the shunt flow increased and drained retrogradely into the perimedullary veins, causing subsequent venous congestion and spinal cord edema, finally resulting in progressive myelopathy.
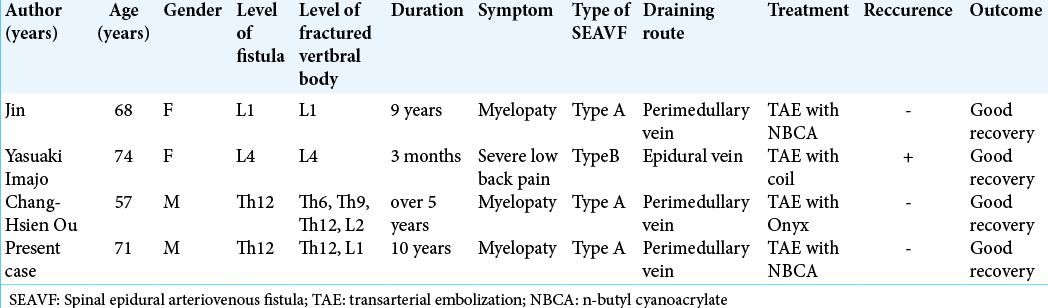
An intraosseous SEAVF with perimedullary reflux, as in this case, is extremely rare, and we were able to confirm only three reports in the literature [
SEAVFs in most cases are located in the ventral epidural space with a shunted pouch.[
SEDAVFs have been treated with several techniques, including surgery, transarterial embolization, transvenous embolization, and combinations of these techniques, but no standard treatment has been established for SEAVFs. More reports of endovascular treatment have been described than direct surgery. In endovascular treatment, the penetration of Onyx or glue into the intradural proximal drainer through the epidural venous pouch is recommended for permanent obliteration of the fistula.[
The present case presented with myelopathy due to spinal congestion caused by Type A SEAVF secondary to a thoracolumbar compression fracture. The fistula was demonstrated in the Th12 vertebral body that had previously experienced a compression fracture, suggesting that vertebral trauma could be associated with the development of shunt formation. Because vertebral compression fractures have been increasing due to population aging, clinicians must be careful to consider the occurrence of an SEAVF as a cause of gradually progressive myelopathy secondary to a vertebral compression fracture.
CONCLUSION
We presented a rare case of an intraosseous SEAVF secondary to a thoracolumbar compression fracture with perimedullary venous reflux causing progressive myelopathy. The fistula was located in the fractured vertebral body.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Hauck EF, Nauta HJ. Spontaneous spinal epidural arteriovenous fistulae in neurofibromatosis Type-1. Surg Neurol. 2006. 66: 215-21
2. Imajo Y, Kanchiku T, Yoshida Y, Nishida N, Taguchi T. Large spinal intraosseous arteriovenous fistula: Case report. J Neurosurg Spine. 2015. 22: 406-8
3. Jin YJ, Chung SK, Kwon OK, Kim HJ. Spinal intraosseous arteriovenous fistula in the fractured vertebral body. Am J Neuroradiol. 2010. 31: 688-90
4. Khaldi A, Hacein-Bey L, Origitano TC. Spinal epidural arteriovenous fistula with late onset perimedullary venous hypertension after lumbar surgery: Case report and discussion of the pathophysiology. Spine (Phila Pa 1976). 2009. 34: 775-9
5. Kiyosue H, Tanoue S, Okahara M, Hori Y, Kashiwagi J, Mori H. Spinal ventral epidural arteriovenous fistulas of the lumbar spine: Angioarchitecture and endovascular treatment. Neuroradiology. 2013. 55: 327-36
6. Murakami T, Nakagawa I, Wada T, Kichikawa K, Nakase H. Lumbar spinal epidural arteriovenous fistula with perimedullary venous drainage after endoscopic lumbar surgery. Interv Neuroradiol. 2015. 21: 249-54
7. Nasr DM, Brinjikji W, Clarke MJ, Lanzino G. Clinical presentation and treatment outcomes of spinal epidural arteriovenous fistulas. J Neurosurg Spine. 2017. 26: 613-20
8. Ou CH, Wang HK, Yang TH, Liang CL, Wong HF. Spinal intraosseous epidural arteriovenous fistula with perimedullary drainage obliterated with Onyx embolization: Case report. J Neurosurg Spine. 2015. 23: 250-3
9. Patsalides A, Knopman J, Santillan A, Tsiouris AJ, Riina H, Gobin YP. Endovascular treatment of spinal arteriovenous lesions: Beyond the dural fistula. Am J Neuroradiol. 2011. 32: 798-808
10. Ramanathan D, Levitt MR, Sekhar LN, Kim LJ, Hallam DK, Ghodke BV. Management of spinal epidural arteriovenous fistulas: Interventional techniques and results. J Neurointerv Surg. 2014. 6: 144-9
11. Rangel-Castilla L, Holman PJ, Krishna C, Trask TW, Klucznik RP, Diaz OM. Spinal extradural arteriovenous fistulas: A clinical and radiological description of different types and their novel treatment with Onyx: Clinical article. J Neurosurg Spine. 2011. 15: 541-9
12. Silva N, Jancel AC, Tall P, Cognard C. Spinal epidural arteriovenous fistulas associated with progressive myelopathy. Report of four cases. J Neurosurg Spine. 2007. 6: 552-8
13. Takai K, Taniguchi M. Comparative analysis of spinal extradural arteriovenous fistulas with or without intradural venous drainage: A systematic literature review. Neurosurg Focus. 2012. 32: E8
14. Takai K, Endo T, Yasuhara T, Seki T, Watanabe K, Tanaka Y. Microsurgical versus endovascular treatment of spinal epidural arteriovenous fistulas with intradural venous drainage: A multicenter study of 81 patients. J Neurosurg Spine. 2020. 33: 381-91
15. Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: Clinical presentation and long-term follow-up in 49 patients. Stroke. 2002. 33: 1578-83