- Department of Neurosurgery, Nagareyama Central Hospital, Chiba, Japan.
- Department of Emergency and Critical Care Medicine, Kansai Medical University General Medical Center, Osaka, Japan.
- Department of Neurosurgery, Medical Center Adachi, Tokyo Women’s Medical University, Tokyo, Japan.
- Department of Neurosurgery, Sassa General Hospital, Nishitokyo, Japan.
Correspondence Address:
Ryuzaburo Kanazawa, Department of Neurosurgery, Nagareyama Central Hospital, Nagareyama, Chiba, Japan.
DOI:10.25259/SNI_625_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryuzaburo Kanazawa1, Tomoyuki Yoshihara2, Takanori Uchida1, Tetsuhiro Higashida1, Naoyuki Arai3, Hidenori Ohbuchi3, Yuichi Takahashi4. Thromboembolic complications during and after embolization of unruptured aneurysms: A chronological outcome in periprocedural thromboembolic events. 13-Oct-2023;14:362
How to cite this URL: Ryuzaburo Kanazawa1, Tomoyuki Yoshihara2, Takanori Uchida1, Tetsuhiro Higashida1, Naoyuki Arai3, Hidenori Ohbuchi3, Yuichi Takahashi4. Thromboembolic complications during and after embolization of unruptured aneurysms: A chronological outcome in periprocedural thromboembolic events. 13-Oct-2023;14:362. Available from: https://surgicalneurologyint.com/surgicalint-articles/12595/
Abstract
Background: Ischemic complications develop after elective coil embolization procedures at a certain rate. The prevention of these events has been a longstanding issue for many interventional neuroradiologists. This study aimed to clarify whether procedural ischemic events after unruptured aneurysm embolization decrease over time with perioperative anti-thromboembolic treatment or surgical experience.
Methods: This study included patients with cerebral aneurysms in our institution between July 2012 and June 2020. Dual-antiplatelet therapy (DAPT) was performed (Phase 1). Thromboembolic events developed at a certain rate; thus, rivaroxaban was administered with single-antiplatelet therapy (SAPT) to improve thromboembolic results (Phase 2), showing better outcomes than in Phase 1. Subsequently, DAPT was administered again (Phase 3). Ischemic complications were evaluated in each phase or compared between the DAPT group and the direct oral anticoagulant (DOAC) with the clopidogrel (DOAC+SAPT) group.
Results: Relatively, fewer symptomatic ischemic events were noted in Phase 2 or the DOAC+SAPT group, but the outcome was not better in Phase 3 than in Phase 2. Symptomatic complications were more common in Phase 3 than in Phases 1 and 2.
Conclusion: Ischemic complications occurred at a certain rate after endovascular procedures for unruptured aneurysms. The incidence did not decrease over time; particularly, standard DAPT plus postoperative anti-thromboembolic medication did not adequately decrease complications in Phase 3 compared to Phases 1 and 2. Therefore, accumulated experience or a learning curve could not explain the results. DOAC administration might decrease the risk of these events, but further accumulation of evidence or prospective investigation is warranted.
Keywords: Direct oral anticoagulants, Dual-antiplatelet therapy, Embolization, Thromboembolic complications, Unruptured aneurysms
INTRODUCTION
The prevention of thromboembolic complications of elective endovascular procedures for cerebral aneurysms is important. A previous study reported that asymptomatic and symptomatic complications occurred in 5.5–70% and 3.8–40% of procedures, respectively.[
In our institution, we previously used conventional dual-antiplatelet therapy (DAPT), but we noted a certain rate of symptomatic thromboembolic events (TEs) (Phase 1). A different medication was administered to reduce TEs (Phase 2), which improved procedural results; then, conventional DAPT was performed to determine whether the result of Phase 2 was reliable, and whether our learning curve was significant (Phase 3). These outcomes were analyzed retrospectively.
MATERIALS AND METHODS
A total of 54 coiling procedures under DAPT from July 2012 to November 2015 (Phase 1), 92 procedures under 75 mg of clopidogrel and 10 or 15 mg of rivaroxaban per day from December 2015 to September 12, 2018 (Phase 2), and 75 procedures under DAPT from September 14, 2018, to June 2020 (Phase 3) were retrospectively compared. In our institution, better results were empirically observed in patients receiving warfarin or direct oral anticoagulant (DOAC) coadministration than in those receiving conventional DAPT with respect to postoperative ischemic events following carotid artery stenting. The ethics committee of our institution was consulted regarding DOAC administration, and it was approved. Three patients in Phase 1 and one patient in Phase 3 were given DOAC and 75 mg of clopidogrel. These patients had a history of nonvalvular atrial fibrillation. In Phase 2, DOAC and 75 mg of clopidogrel were administered to 55 patients, whereas 27 patients who refused coadministration received DAPT.
All patients in the DAPT group received 100 mg of aspirin and 75 mg of clopidogrel daily. The medications were started >1 week before the procedure.[
All cases of elective embolization for unruptured saccular or fusiform aneurysms in the present study and re-operation for recanalization of ruptured aneurysms were included in the study. Simultaneous treatment of two or more aneurysms, procedures for coincident unruptured aneurysms in the acute stage of subarachnoid hemorrhage, and procedures using a flow diversion stent were excluded from the study.
Basically, during the procedure and the entire follow-up period, systolic blood pressure and diastolic blood pressure were controlled to <140 mmHg and <90 mmHg, respectively. Systemic heparinization was performed according to the activated clotting time and maintained from 200 s to 300 s. The addition of an optional anti-thromboembolic agent postoperatively (intravenous continuous infusion of heparin [10,000 U/day], ozagrel sodium [80 mg twice a day], or argatroban hydrate [60 mg/day]) was permitted at the operator’s discretion.
No platelet aggregation test was performed in this study because platelet aggregation measurement was not available in our institution until June 2020.
All procedures were conducted after patients provided written, informed consent. The ethics committee of our institution approved the prescription of perioperative DOAC, and the study followed the principles outlined in the Declaration of Helsinki.
Evaluation of ischemic complications
Diffusion-weighted imaging (DWI) and magnetic resonance angiography were performed in all patients within 72 h after the procedure. Imaging was performed with a 1.5-T system (Infinix INFX-8000V; Canon, Tokyo, Japan). Each high-intensity signal was counted in every DWI slice. The total count was designated the “DWI count” [
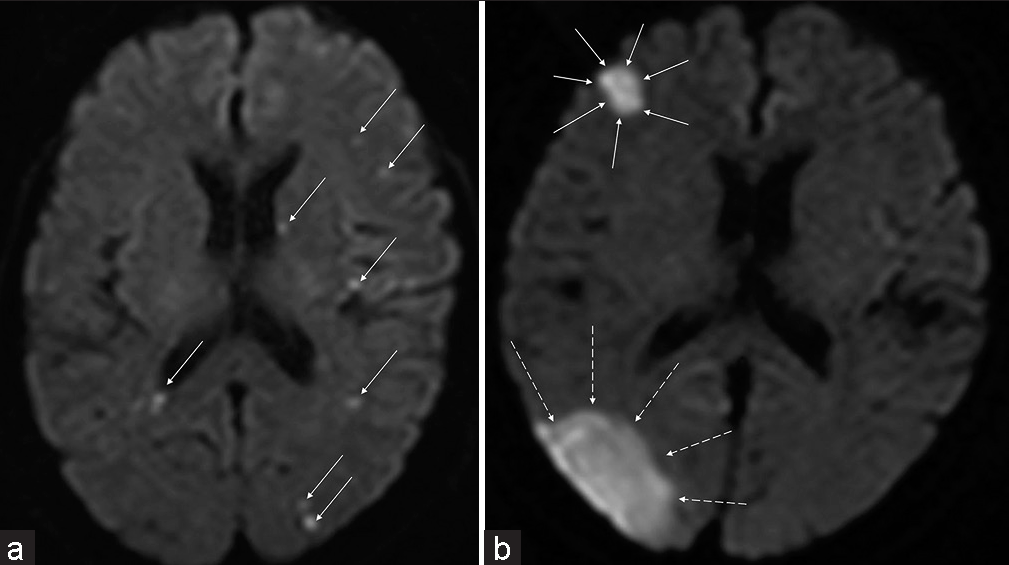
Figure 1:
Method of calculating the diffusion-weighted imaging (DWI) score. Every high-intensity DWI spot (a: each indicated by an arrow) or area (b: surrounded by arrows or dotted arrows) is calculated as 1; every spot (a) is counted in each image slice, but a DWI area (b) exists over multiple slices; thus, the whole lesion over slices is counted as 1. The sum of these DWI findings is defined as the “DWI count.”
Measurement of aneurysm size
Maximum aneurysmal diameter (Dmax) and neck and parent artery diameter (PAD) were measured on three-dimensional digital subtraction angiography, and the dome and neck ratio (D/N ratio) was calculated from Dmax/neck.
Statistical analysis
Each parameter (age, total amount of heparin, operation time, aneurysmal neck [neck], PAD, Dmax, and D/N ratio) was analyzed in each phase using a one-way analysis of variance (ANOVA). In the comparison of phases, the analysis was based on Phase 1. Comparisons of the same parameters between the DAPT and DOAC+SAPT groups were performed with the unpaired t-test. An analysis of DWI count was also performed to evaluate whether improvement in ischemic events was achieved over time across the three phases and to identify a difference between the DAPT and DOAC+SAPT groups. The number of lesions >10 mm was analyzed in each phase or compared between the DAPT and DOAC+SAPT groups using the Chi-squared test, and if the data were significant, residuals were analyzed. Logistic regression analysis was used to evaluate factors affecting DWI-positive or DWI-negative status and symptomatic complications, and multiple regression analysis was used for the DWI count. These multivariate analyses were conducted in all cases and in the DAPT and DOAC+SAPT groups because it was possible that different factors could affect ischemic events with different medications.
P < 0.05 was considered significant. Bell Curve for Excel version 3.22 (SSRI, Tokyo, Japan) was used for the analyses.
RESULTS
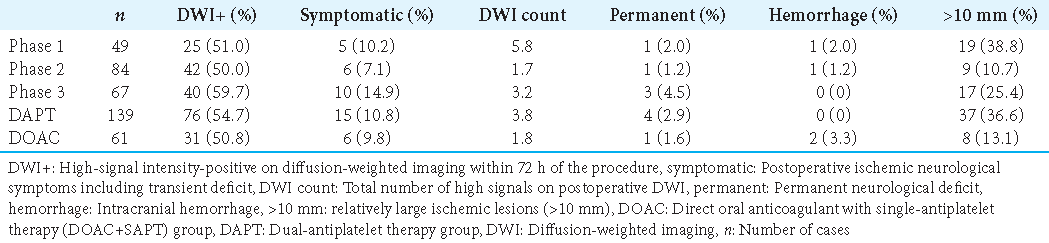
A total of 200 patients were included in the present study: 49 in Phase 1, 84 in Phase 2, and 67 in Phase 3. Of these, 14 (28.6%), 27 (32.1%), and 14 (20.9%) in each phase and 34 (24.5%) in the DAPT group and 21 (34.4%) in the DOAC+SAPT group were male. Moreover, 139 patients received periprocedural DAPT (DAPT group), and 61 received DOAC and clopidogrel (DOAC+SAPT group). Additional postoperative antiplatelet or anticoagulation treatment was performed in 7 (14.3%) patients in Phase 1, 6 (7.1%) patients in Phase 2, and 19 (28.4%) patients in Phase 3 within 24 h after the procedure.
Summary of aneurysm location
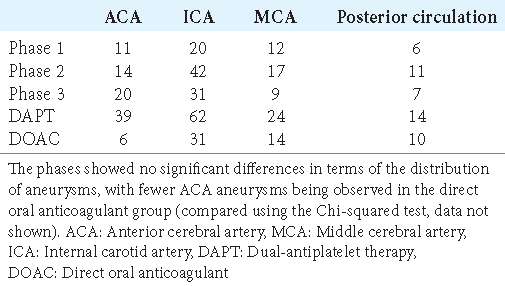
Aneurysm locations are summarized in
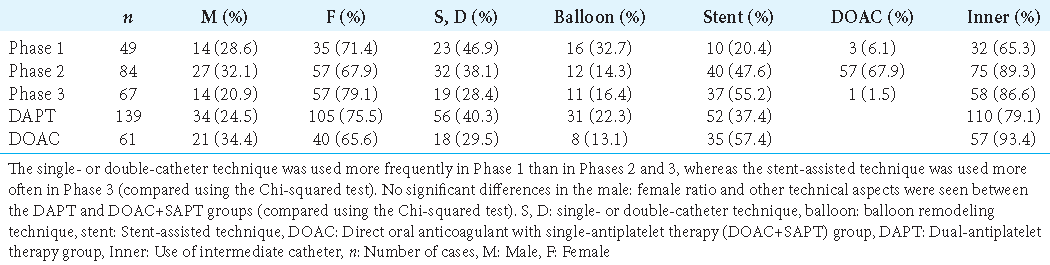
Comparison of procedural techniques in each phase and between the two groups
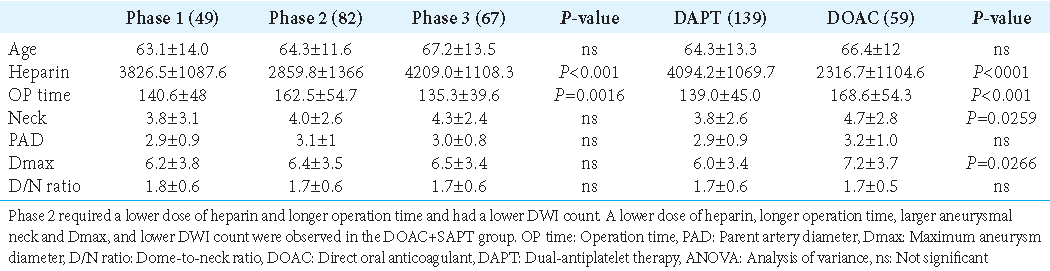
Comparison of parameters among phases (one-way ANOVA) and between the DAPT and DOAC groups
The amount of heparin used during the procedure was significantly lower in Phase 2 (P < 0.001), and the operation time was significantly longer in Phase 2 (P = 0.0016) [
Neck size and Dmax were significantly larger in the DOAC+SAPT group than in the DAPT group.
Ischemic complications and permanent deficit
Neurological symptoms occurred within 1 day postoperatively in 20 of 21 (95.2%) patients.
Postoperative ischemic lesions >10 mm on DWI
Complications not of thromboembolic origin
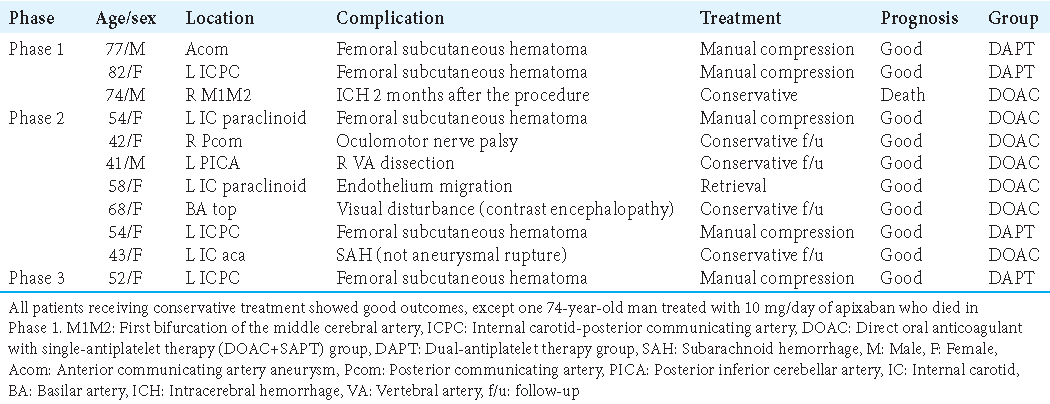
In this study, 11 patients developed complications not of thromboembolic origin [
Factors related to high-intensity DWI signals and symptomatic complications (multivariate analysis)
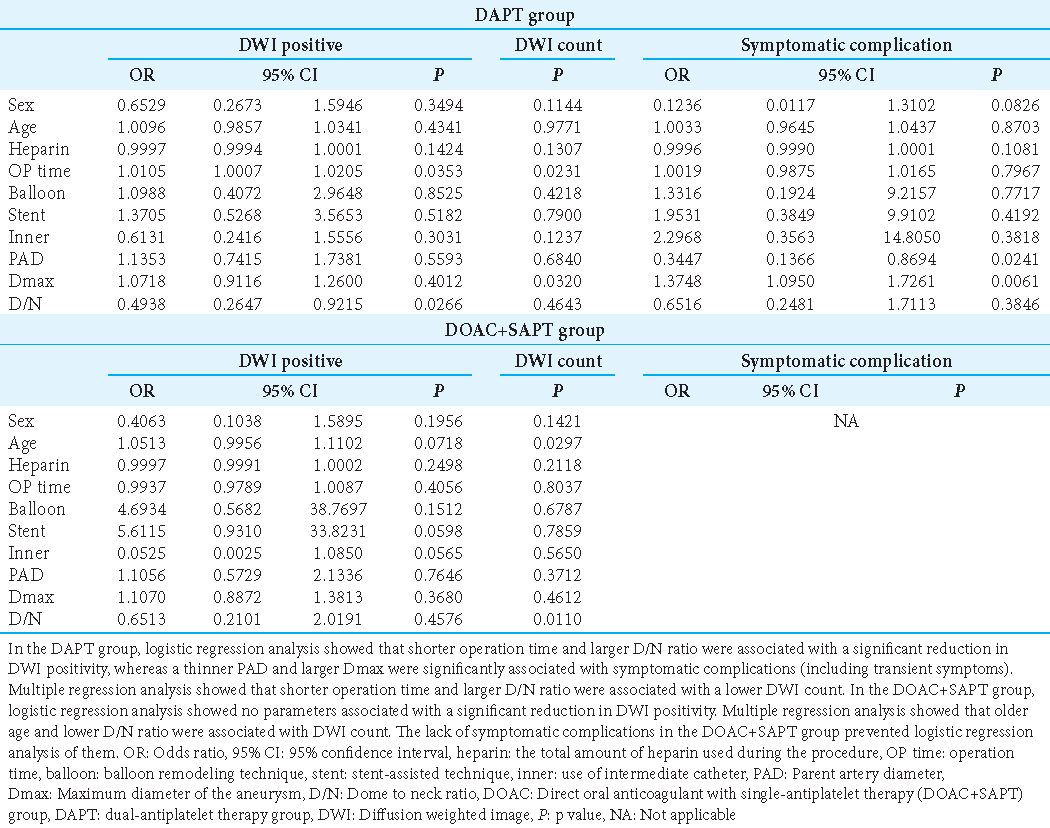
These analyses were conducted among DAPT and DOAC+SAPT cases [
Patients receiving DAPT (n = 139)
DWI-positive findings were significantly affected by operation time and the D/N ratio. The DWI score was significantly related to operation time and Dmax. PAD and Dmax were significantly related to symptomatic complications.
Patients receiving DOAC (n = 61)
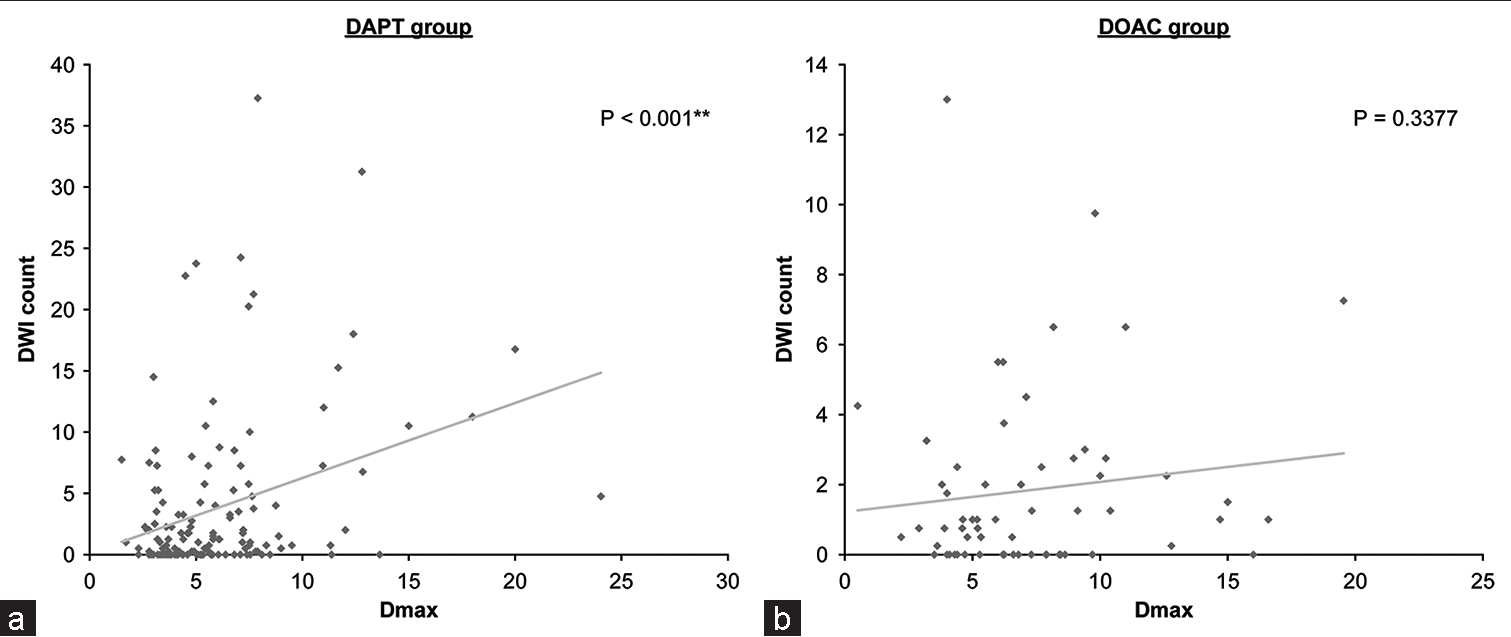
DWI-positive findings were not significantly affected by each parameter. The DWI score was significantly related to age and the D/N ratio. The number of symptomatic complication cases was insufficient for statistical analysis. Patients administered DAPT had a larger aneurysm size and higher DWI counts (P < 0.001), whereas a similar tendency was not seen in patients on DOAC (P = 0.3377;
Figure 2:
Relationship between diffusion-weighted imaging (DWI) count and aneurysmal maximum aneurysm diameter. (a) The regression analysis of the relationship between diffusion-weighted imaging (DWI) count (vertical axis) and Dmax (horizontal axis) shows significance in the dual-antipletelet therapy group. (b) The relationship between these parameters is not significant in the direct oral anticoagulation with single-antipletelet therapy group.
DISCUSSION
Due to improvements in various devices, the number of endovascular procedures performed for cerebral aneurysms is increasing. The administration of perioperative anti-thromboembolic agents helps prevent ischemic events.[
In the present study, 50–60% of cases in each phase showed DWI-positive lesions and symptomatic ischemic events occurred in 10.2% of cases in Phase 1, 6% in Phase 2, and 14.9% in Phase 3. All procedures were performed by experienced neuroradiologists. Unfortunately, the learning curve did not always contribute to reducing ischemic events, although the DWI count was lower in Phase 3 than in Phase 1, which may be attributed to the efficacy of postoperative optional antiplatelet or anticoagulant agents. More symptomatic complications were observed in Phase 3 than in Phase 1 or 2; therefore, the problem remains, although most ischemic complications were reversible.
Periprocedural antiplatelet treatment with systemic heparinization has been generally accepted in coil embolization. The efficacy of systemic heparinization or perioperative antiplatelet treatment has been shown by several authors.[
Brooks et al. reported that silent and symptomatic ischemia occurred in 43–61% and 3.8–40% of aneurysmal coil embolization procedures with DAPT and systemic heparinization, respectively.[
Nishikawa et al. found that DAPT was more effective than SAPT (aspirin) for wide-necked aneurysms.[
In general, postoperative high signal intensity on DWI was noted in 10.4–64% of cases,[
In the present study, DWI findings were positive in 50–59.7% of cases through all phases, and symptomatic ischemic events, including transient symptoms, were observed in 6–14.9%. The present results were compatible with those of the previous studies, but the experience did not result in better outcomes than before, which could be interpreted as a result of the more complicated technique, larger neck, and greater aneurysmal size as a target compared with the previous reports.[
Conversely, DOAC can possibly reduce ischemic events in aneurysmal coiling. Fewer DWI-positive findings, lower DWI count, fewer large lesions (>10 mm), and fewer symptomatic ischemic complications were seen in the DOAC+SAPT group, despite the larger aneurysmal neck size and longer operation time. Many studies have emphasized aneurysmal size, wider neck, and longer operative time as factors related to ischemic events,[
In Phase 2, rivaroxaban was used to prevent ischemic complications in aneurysmal coiling procedures. It was interesting that there were no parameters related to DWI positivity in the DOAC group. It was remarkable that the DWI count in the DOAC group was not significantly related to aneurysmal size.
Rivaroxaban is a direct factor Xa inhibitor that may provide more consistent and predictable anticoagulation than warfarin,[
Limitations
This was a retrospective study with extremely limited evidence. The antiplatelet aggregation function was not evaluated. Although a comparatively low ischemic complication rate was seen in patients administered DOAC, the reason that similar results were not achieved with suitable heparin administration remains unclear. Fewer patients receiving DOAC were treated for anterior communicating artery aneurysms than patients receiving DAPT. The present study was also subject to bias in patient selection during the acquisition of informed consent. Finally, magnetic resonance imaging was not always performed immediately before the procedures in all patients, which might be the reason that findings on postoperative DWI were not completely identified during or after the intervention.
CONCLUSION
Compared with the findings of the previous studies, the present results for unruptured cerebral aneurysm coiling in relation to periprocedural thromboembolic complications were acceptable. However, the learning curve did not resolve the longstanding issue of reducing embolic events during or after aneurysmal embolization procedures. DOAC administration might be a possible solution for this issue, but large-scale and prospective studies are needed to confirm the present data.
Ethical approval
The author(s) declare that they have taken the ethical approval from IRB/IEC.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that they have used artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript or image creations.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors would like to thank the radiologists at their hospital for their dedicated assistance during the procedures. The authors also express their heartfelt thanks to the one neurosurgeon (Hidetoshi Shimaguchi) and the two neurologists (Tomohisa Dembo and Takashi Tajima) who assisted us in the interpretation of several diffusion-weighted images.
References
1. Albayram S, Selcuk H, Kara B, Bozdag E, Uzma O, Kocer N. Thromboembolic events associated with balloon-assisted coil embolization: Evaluation with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2004. 25: 1768-77
2. Almekhlafi MA, Al Sultan AS, Kuczynski AM, Brinjikji W, Menon BK, Hill MD. Antiplatelet therapy for prevention of thromboembolic complications in coiling-only procedures for unruptured brain aneurysms. J Neurointerv Surg. 2020. 12: 298-302
3. Bond KM, Brinjikji W, Murad MH, Kallmes DF, Cloft HJ, Lanzino G. Diffusion-weighted imaging-detected ischemic lesions following endovascular treatment of cerebral aneurysms: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017. 38: 304-9
4. Brooks NP, Turk AS, Niemann DB, Aagaard-Kienitz B, Pulfer K, Cook T. Frequency of thromboembolic events associated with endovascular aneurysm treatment: Retrospective case series. J Neurosurg. 2008. 108: 1095-100
5. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013. 381: 1107-15
6. Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004. 364: 331-7
7. Ezekowitz MD, Pollack CV, Halperin JL, England RD, VanPelt Nguyen S, Spahr J. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: The EMANATE trial. Eur Heart J. 2018. 39: 2959-71
8. Flierl U, Fraccarollo D, Micka J, Bauersachs J, Schäfer A. The direct factor Xa inhibitor Rivaroxaban reduces platelet activation in congestive heart failure. Pharmacol Res. 2013. 74: 49-55
9. Hahnemann ML, Ringelstein A, Sandalcioglu IE, Goericke S, Moenninghoff C, Wanke I. Silent embolism after stent-assisted coiling of cerebral aneurysms: Diffusion-weighted MRI study of 75 cases. J Neurointerv Surg. 2014. 6: 461-5
10. Higashi E, Matsumoto S, Nakahara I, Hatano T, Ishii A, Sadamasa N. Clopidogrel response predicts thromboembolic events associated with coil embolization of unruptured intracranial aneurysms: A prospective cohort study. PLoS One. 2021. 16: e0249766
11. Hwang G, Huh W, Lee JS, Villavicencio JB, Villamor RB, Ahn SY. Standard vs modified antiplatelet preparation for preventing thromboembolic events in patients with high on-treatment platelet reactivity undergoing coil embolization for an unruptured intracranial aneurysm: A randomized clinical trial. JAMA Neurol. 2015. 72: 764-72
12. Hwang G, Jung C, Park SQ, Kang HS, Lee SH, Oh CW. Thromboembolic complications of elective coil embolization of unruptured aneurysms: The effect of oral antiplatelet preparation on periprocedural thromboembolic complication. Neurosurgery. 2010. 67: 743-8
13. Iosif C, Lecomte JC, Pedrolo-Silveira E, Mendes G, Boncoeur Martel MP, Saleme S. Evaluation of ischemic lesion prevalence after endovascular treatment of intracranial aneurysms, as documented by 3-T diffusion-weighted imaging: A 2-year, single-center cohort study. J Neurosurg. 2018. 128: 982-91
14. Ishihara H, Ishihara S, Niimi J, Neki H, Kakehi Y, Uemiya N. Risk factors for coil protrusion into the parent artery and associated thrombo-embolic events following unruptured cerebral aneurysm embolization. Interv Neuroradiol. 2015. 21: 178-83
15. Jo KI, Yeon JY, Kim KH, Jeon P, Kim JS, Hong SC. Predictors of thromboembolism during coil embolization in patients with unruptured intracranial aneurysm. Acta Neurochir (Wien). 2013. 155: 1101-6
16. Kim MS, Park ES, Park JB, Lyo IU, Sim HB, Kwon SC. Clopidogrel response variability in unruptured intracranial aneurysm patients treated with stent-assisted endovascular coil embolization: Is follow-up clopidogrel response test necessary?. J Korean Neurosurg Soc. 2018. 61: 201-11
17. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Safety, tolerability, pharmacodynamics, and pharmacokinetics of Rivaroxaban--an oral, direct factor Xa inhibitor--are not affected by aspirin. J Clin Pharmacol. 2006. 46: 981-90
18. Kubitza D, Becka M, Mück W, Schwers S. Effect of co-administration of rivaroxaban and clopidogrel on bleeding time, pharmacodynamics and pharmacokinetics: A phase I study. Pharmaceuticals (Basel). 2012. 5: 279-96
19. Li W, Zhu W, Wang A, Zhang G, Zhang Y, Wang K. Effect of adjusted antiplatelet therapy on preventing ischemic events after stenting for intracranial aneurysms. Stroke. 2021. 52: 3815-25
20. Matsumoto Y, Kondo R, Matsumori Y, Shimizu H, Takahashi A, Tominaga T. Antiplatelet therapy for prevention of thromboembolic complications associated with coil embolization of unruptured cerebral aneurysms. Drugs R D. 2012. 12: 1-7
21. Nishikawa Y, Satow T, Takagi T, Murao K, Miyamoto S, Iihara K. Efficacy and safety of single versus dual antiplatelet therapy for coiling of unruptured aneurysms. J Stroke Cerebrovasc Dis. 2013. 22: 650-5
22. Park JC, Lee DH, Kim JK, Ahn JS, Kwun BD, Kim DY. Microembolism after endovascular coiling of unruptured cerebral aneurysms: Incidence and risk factors. J Neurosurg. 2016. 124: 777-83
23. Park SH, Kim YB, Huh SK. Effect of premedication method and drug resistance of antiplatelet agent on periprocedural thromboembolic events during coil embolization of an unruptured intracranial aneurysm. J Cerebrovasc Endovasc Neurosurg. 2012. 14: 148-56
24. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011. 365: 883-91
25. Pierot L, Barbe C, Nguyen HA, Herbreteau D, Gauvrit JY, Januel AC. Intraoperative complications of endovascular treatment of intracranial aneurysms with coiling or balloon-assisted coiling in a prospective multicenter cohort of 1088 participants: Analysis of recanalization after endovascular treatment of intracranial aneurysm (ARETA) study. Radiology. 2020. 295: 381-9
26. Platz J, Wagner M, Güresir E, You SJ, Konczalla J, de Rochemont RD. Early diffusion-weighted MRI lesions after treatment of unruptured intracranial aneurysms: A prospective study. J Neurosurg. 2017. 126: 1070-8
27. Rahme RJ, Zammar SG, El Ahmadieh TY, El Tecle NE, Ansari SA, Bendok BR. The role of antiplatelet therapy in aneurysm coiling. Neurol Res. 2014. 36: 383-8
28. Ries T, Buhk JH, Kucinski T, Goebell E, Grzyska U, Zeumer H. Intravenous administration of acetylsalicylic acid during endovascular treatment of cerebral aneurysms reduces the rate of thromboembolic events. Stroke. 2006. 37: 1816-21
29. Sedat J, Chau Y, Gaudart J, Sachet M, Beuil S, Lonjon M. Prasugrel versus clopidogrel in stent-assisted coil embolization of unruptured intracranial aneurysms. Interv Neuroradiol. 2017. 23: 52-9
30. Seo DH, Yoon SM, Park HR, Shim JJ, Bae HG, Yun IG. Thromboembolic event detected by diffusion weighted magnetic resonance imaging after coil embolization of cerebral aneurysms. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 175-83
31. Sotomi Y, Hirata A, Amiya R, Kobayashi T, Hirayama A, Sakata Y. Bleeding risk of add-on anti-platelet agents to direct oral anticoagulants in patients with nonvalvular atrial fibrillation (From 2216 patients in the DIRECT registry). Am J Cardiol. 2019. 123: 1293-300
32. Takigawa T, Suzuki K, Sugiura Y, Suzuki R, Takano I, Shimizu N. Thromboembolic events associated with single balloon-, double balloon-, and stent-assisted coil embolization of asymptomatic unruptured cerebral aneurysms: Evaluation with diffusion-weighted MR imaging. Neuroradiology. 2014. 56: 1079-86
33. Tokunaga K, Hatano T, Nakahara I, Ishii A, Higashi E, Kamata T. Factors associated with postprocedural diffusion-weighted imaging-positive lesions in endovascular treatment for unruptured cerebral aneurysms. World Neurosurg. 2019. 130: e457-62
34. Warnock LB, Huang D, editors. Heparin. StatPearls. Treasure Island, FL: StatPearls Publishing; 2021. p.
35. Wilson TJ, Pandey AS, Stetler WR, Davis MC, Giles DA, Chaudhary N. Dual antiplatelet therapy plus postoperative heparin and dextran is safe and effective for reducing risk of embolic stroke during aneurysm coiling. Acta Neurochir (Wien). 2014. 156: 855-9
36. Yamada NK, Cross DT, Pilgram TK, Moran CJ, Derdeyn CP, Dacey RG. Effect of antiplatelet therapy on thromboembolic complications of elective coil embolization of cerebral aneurysms. AJNR Am J Neuroradiol. 2007. 28: 1778-82
37. Yasaka M, Minematsu K, Toyoda K, Mori E, Hirano T, Hamasaki T. Rivaroxaban administration after acute ischemic stroke: The RELAXED study. PLoS One. 2019. 14: e0212354