- Department of Surgical Neurology, Research Institute for Brain and Blood Vessels Akita, Akita, Japan
Correspondence Address:
Tatsuya Ishikawa
Department of Surgical Neurology, Research Institute for Brain and Blood Vessels Akita, Akita, Japan
DOI:10.4103/2152-7806.173570
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Okada T, Ishikawa T, Moroi J, Suzuki A. Timing of retreatment for patients with previously coiled or clipped intracranial aneurysms: Analysis of 156 patients with multiple treatments. Surg Neurol Int 07-Jan-2016;7:
How to cite this URL: Okada T, Ishikawa T, Moroi J, Suzuki A. Timing of retreatment for patients with previously coiled or clipped intracranial aneurysms: Analysis of 156 patients with multiple treatments. Surg Neurol Int 07-Jan-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/timing-of-retreatment-for-patients-with-previously-coiled-or-clipped-intracranial-aneurysms-analysis-of-156-patients-with-multiple-treatments/
Abstract
Background:Some patients require a second surgical intervention for recurrence of treated aneurysms, untreated aneurysms in patients with multiple lesions, or de novo aneurysm. This retrospective review of the data was undertaken to evaluate when retreatment is necessary after initial aneurysm treatment.
Methods:Cerebral aneurysms in 1755 patients were treated via clipping or coiling between January 1995 and September 2012. Postoperative follow-up was performed at 6 months after treatment and was repeated every 12 months (or longer) after treatment using three-dimensional computed tomography angiography or magnetic resonance angiography.
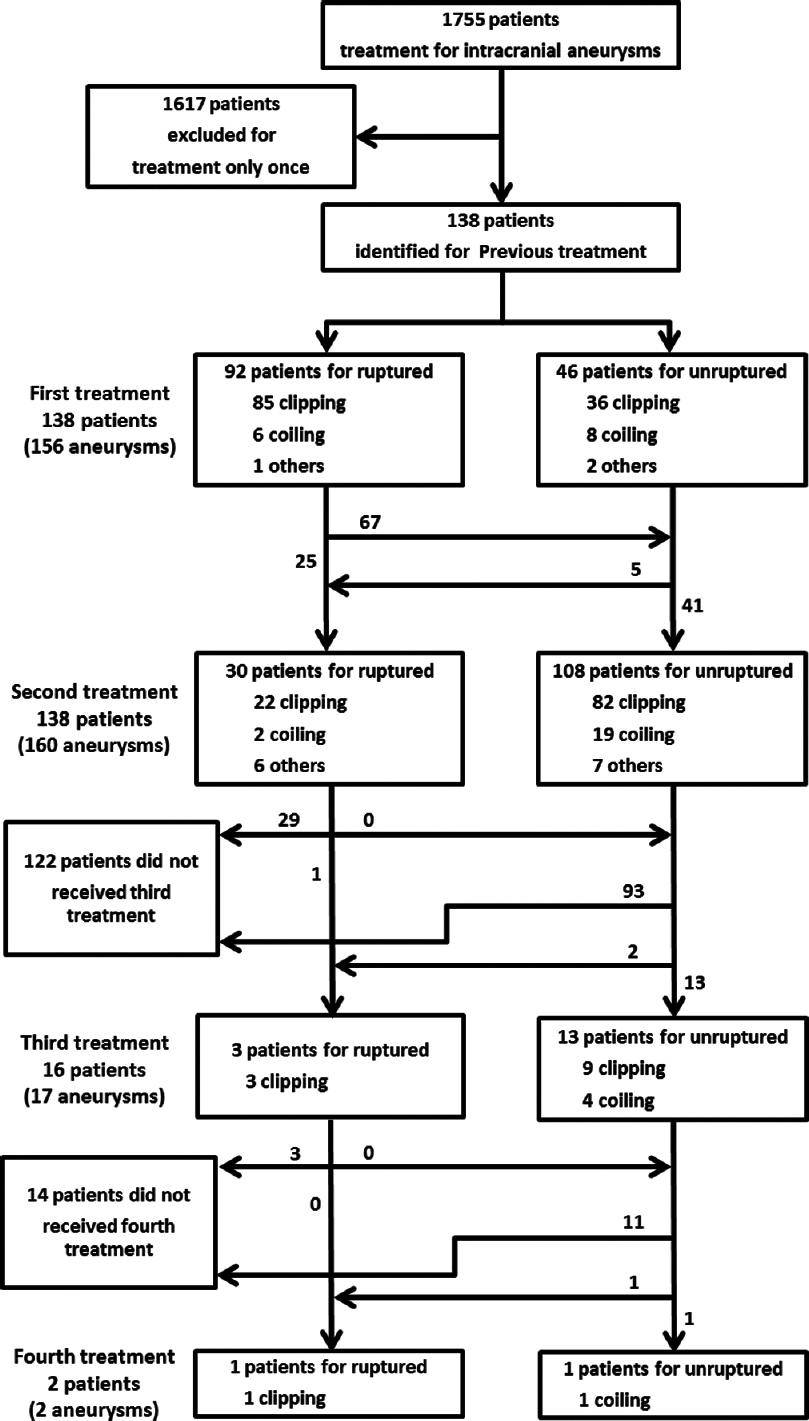
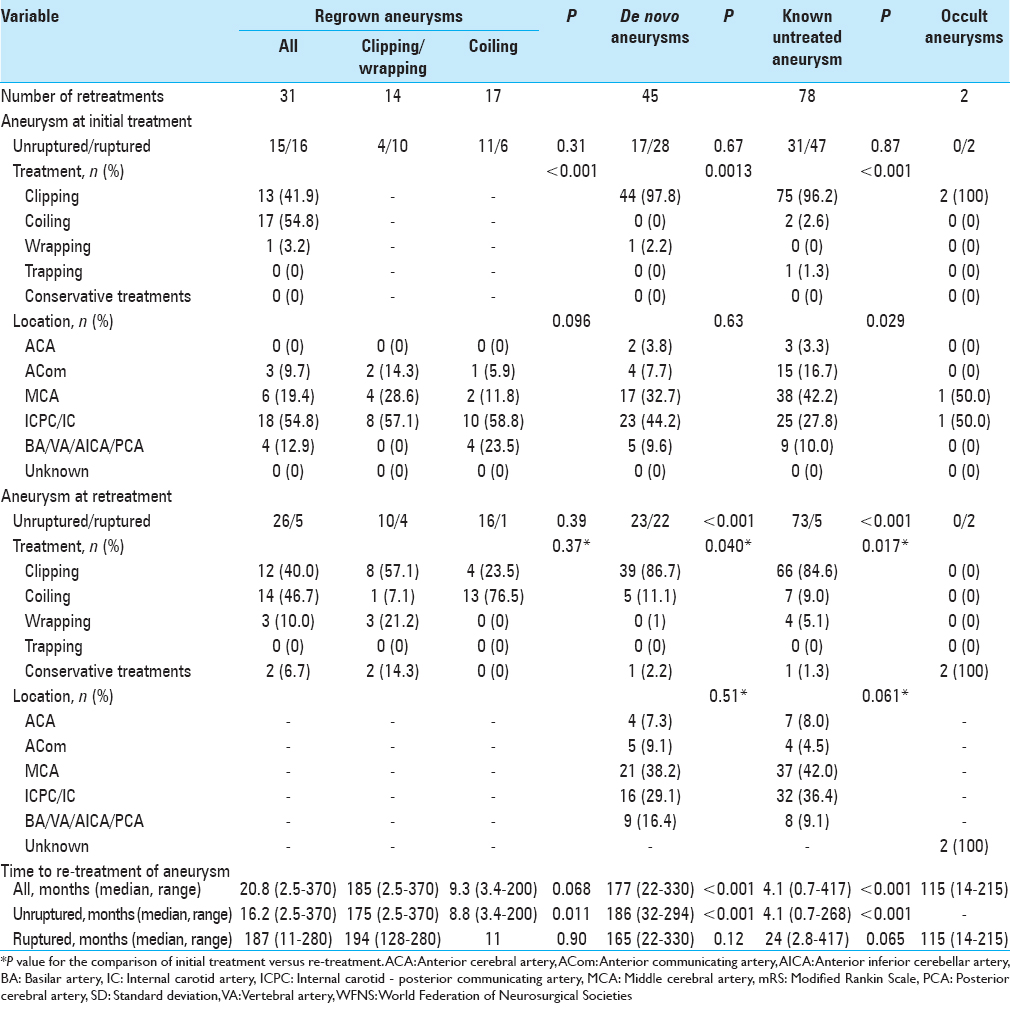
Results:A cumulative total of 156 patients (8.9%) (117 women, 39 men; mean age: 55.0 years; range: 25–79 years) needed retreatment for rupture or regrowth of aneurysm (n = 31; ruptured (R)/remaining unruptured (U), 26/5), formation of de novo aneurysm (n = 45; R/U, 23/22), known untreated aneurysm in patients with multiple lesions (n = 78; R/U, 5/73), and hemorrhage from undetected aneurysm (n = 2). The regrowth risk is higher after endovascular treatment than after craniotomy and clipping. Median time to retreatment was 187 months (range: 11–280 months) for regrowth, 165 months (range: 22–330 months) for de novo, and 24 months (range: 2.8–417 months) for known untreated aneurysm. Regrowth or known with subarachnoid hemorrhage were frequently treated within 2 years from initial treatment.
Conclusions:Aneurysms with residua or untreated aneurysms in patients with multiple lesions carry a risk of bleeding during a relatively short period, whereas there is a small but significant risk of de novo formation and subsequent hemorrhage at over 10 years after previous treatment.
Keywords: De novo aneurysm, follow-up, intracranial aneurysm, regrown aneurysm, retreatment
INTRODUCTION
Aneurysm clipping and coil embolization have provided robust treatments for preventing rupture of intracranial aneurysm. However, some patients must undergo retreatment due to recurrent aneurysm at the originally treated site, de novo aneurysm at a remote location, or aneurysms left untreated at initial treatment in cases of multiple aneurysms. Several case series have suggested that the risk of intracranial aneurysm formation is relatively high after surgical treatment.[
MATERIALS AND METHODS
Patients and data collection
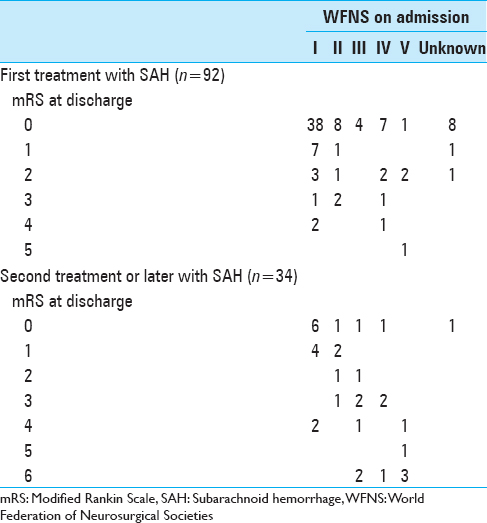
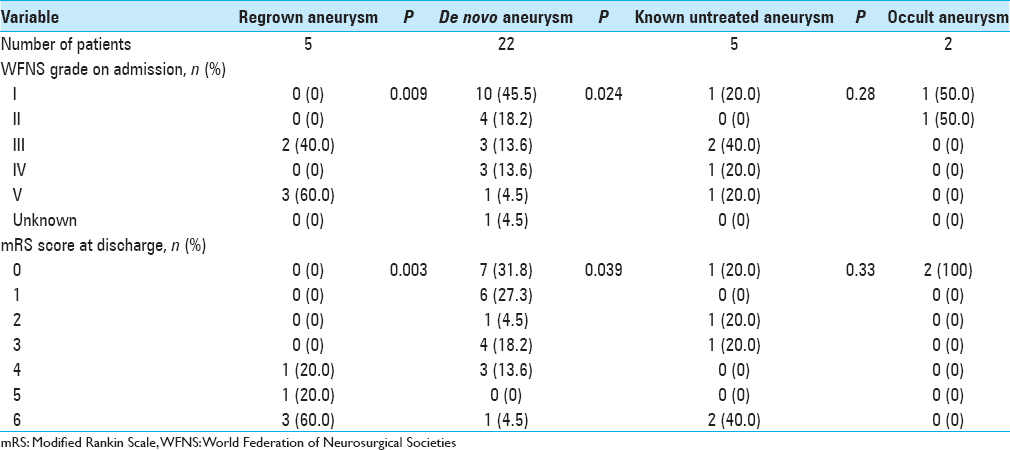
The Institutional Medical Review Board of the Research Institute for Brain and Blood Vessels–Akita approved the study protocol. From January 1995 to December 2012, a total of 1755 patients were admitted to the hospital for treatment of ruptured or unruptured intracranial aneurysms. Of those, we retrospectively identified those patients who had previously been admitted for surgical or endovascular obliteration treatments for intracranial aneurysms. In those patients, retreatment had been performed for the following reasons: Aneurysm located at the same site as the originally treated one, representing regrowth and/or rupture (regrown aneurysm); aneurysm located at a site completely remote from the originally treated one, is identified and/or ruptures (de novo aneurysm); aneurysm that had been visible at initial treatment, but had been left untreated (known untreated aneurysm); or subarachnoid hemorrhage (SAH) of unknown cause (occult aneurysm). We obtained information from medical records and operation records to assess clinical characteristics, the interval between initial treatment and retreatment, and outcomes for patients according to the type of retreatment. Clinical outcomes for patients with ruptured aneurysm were defined using modified Rankin scale (mRS) score at discharge.
Follow-up interval and acquisition of diagnostic images
Follow-up diagnosis of aneurysm for patients who underwent surgical clipping was based on images obtained using three-dimensional computed tomography angiography (3D-CTA) at least once within 6 months of the initial treatment. Magnetic resonance angiography (MRA) was performed every 12 months thereafter until 2 years after surgery. Patients were also repeatedly examined with 3D-CTA or MRA every 1–5 years. Particularly in patients with suspicious residua or regrowth of the treated aneurysm, 3D-CTA was repeated frequently. Diagnostic images for patients who underwent endovascular coiling were obtained using plain skull X-ray to evaluate the formation of coils at least once within 1 month of initial treatment, plain skull X-ray and MRA once within 6 months, digital subtraction angiography (DSA) once around 12 months, and plain skull X-ray and MRA once every 12 months thereafter. If these patients were admitted for retreatment of the intracranial aneurysm, diagnostic imaging was performed on admission and postoperatively using 3D-CTA, MRA, and/or DSA depending on the treatments that had been performed initially and at retreatment.
Indications for surgical or endovascular intervention of intracranial aneurysms
Regardless of the treatment either the 1st time or on retreatment, we treated all patients with ruptured aneurysms defined as the World Federation of Neurosurgical Societies (WFNS) Grades I–IV, as well as selected patients with WFNS Grade V, who should have at least one of the following conditions: Glasgow Coma Scale score > 6; improving neurology; or large hematoma formation associated with SAH. We recommended surgical or endovascular treatment for patients with de novo or known untreated aneurysms that remained unruptured, when they had a history of another ruptured aneurysm, aneurysm larger than 5 mm, irregularly shaped, or that transformed in shape during follow-up. We took both age and activities of daily living (ADL) of the patient into consideration for treatment. We offered treatment to patients with regrown aneurysm, unless a significant contraindication was present.
Statistical analysis
Statistical analyses were performed using SPSS for Windows version 21.0 (SPSS Japan, Tokyo, Japan). Categorical variables, except for the method of surgical or endovascular intervention at retreatment and location of the aneurysm at retreatment, were compared between the target group and other groups using Fisher's exact test or the Pearson Chi-square test. Methods of surgical or endovascular intervention at retreatment and locations of aneurysm at retreatment were compared to those at initial treatment using Fisher's exact test. Continuous variables were compared between the target group and other groups using the Mann–Whitney U-test or Student's t-test. Missing data were omitted and analyses of the available data were performed. Two-sided values of P < 0.05 were considered statistically significant.
RESULTS
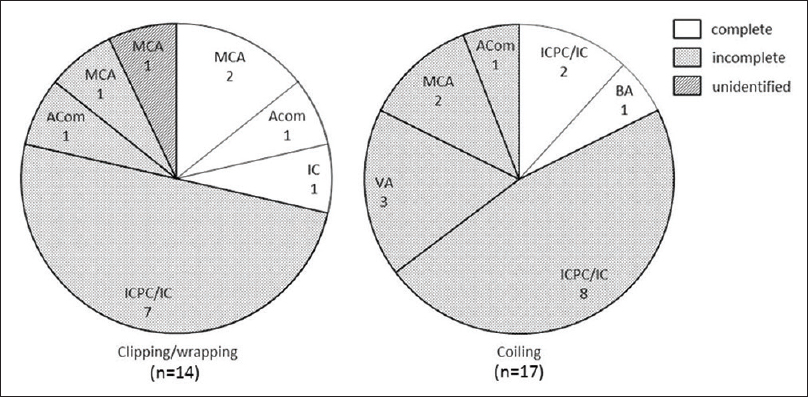
Figure 2
Pie graphs showing the proportion of regrown aneurysm locations by degree of occlusion at initial treatment. ACom: Anterior communicating artery, BA: Basilar artery, IC: Internal carotid artery, ICPC: Internal carotid-posterior communicating artery, MCA: Middle cerebral artery, and VA: Vertebral artery
In patients with retreatment performed for aneurysmal bleeding, median time from initial treatment to retreatment was 165 months (range: 22–330 months) for de novo aneurysm, 187 months (range: 11–280 months) for regrown aneurysm, and 24 months (range: 2.8–417 months) for known untreated aneurysm [
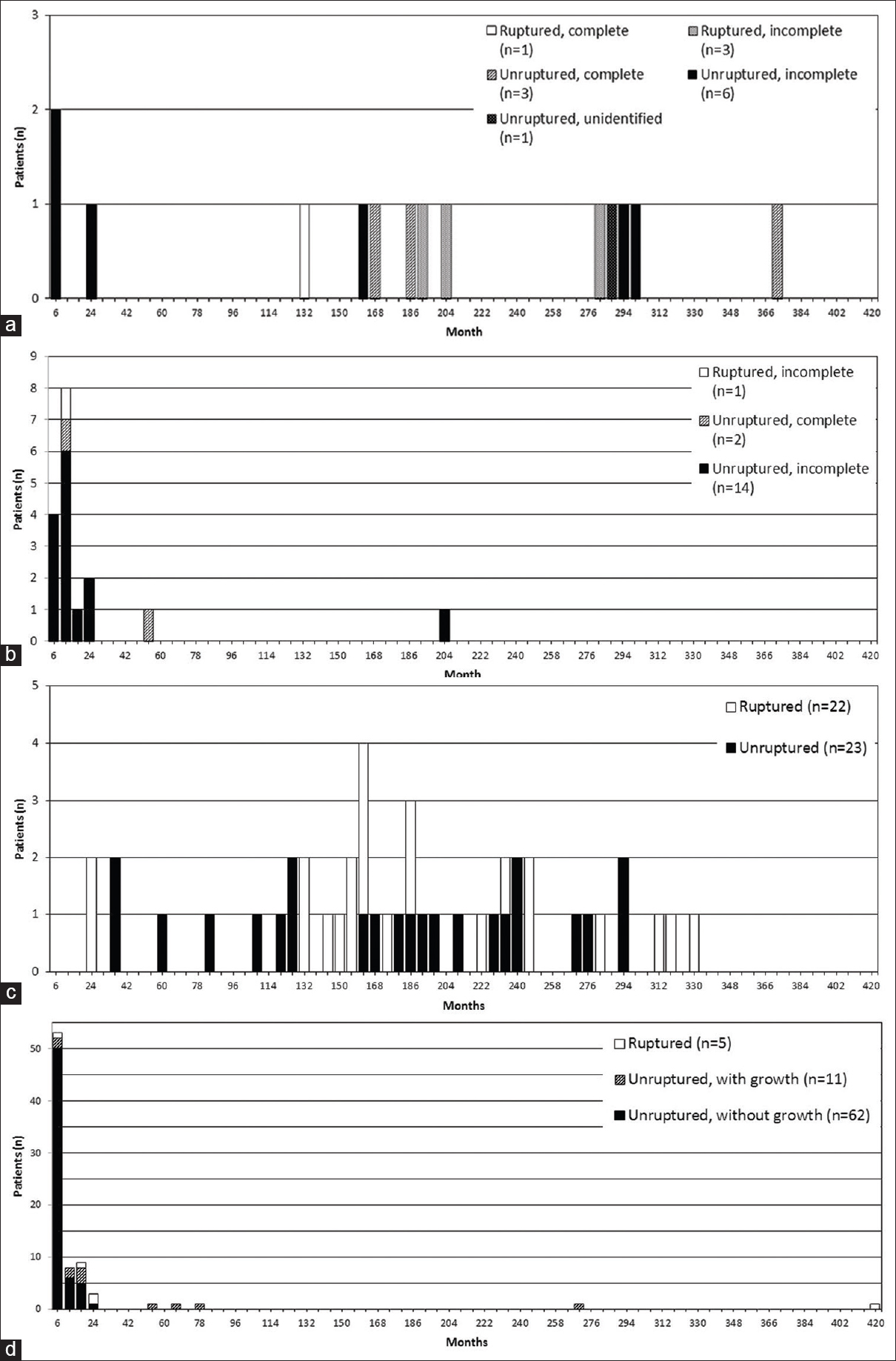
Figure 3
Bar graphs showing interval between initial treatment and retreatment by type of recurrence. (a and b) Regrown aneurysms treated by clipping (a) and coil embolization (b) at initial treatment according to aneurysms with and without rupture at retreatment, and aneurysms with and without incomplete obliteration at initial treatment. (c) De novo aneurysms according to aneurysms with and without rupture at retreatment. (d) Known untreated aneurysms according to aneurysms with and without rupture at retreatment, and change in size
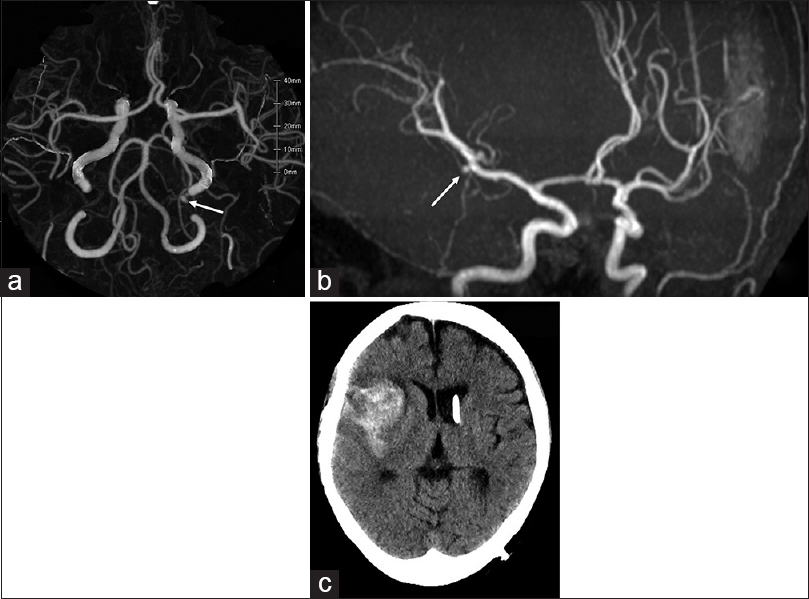
Figure 4
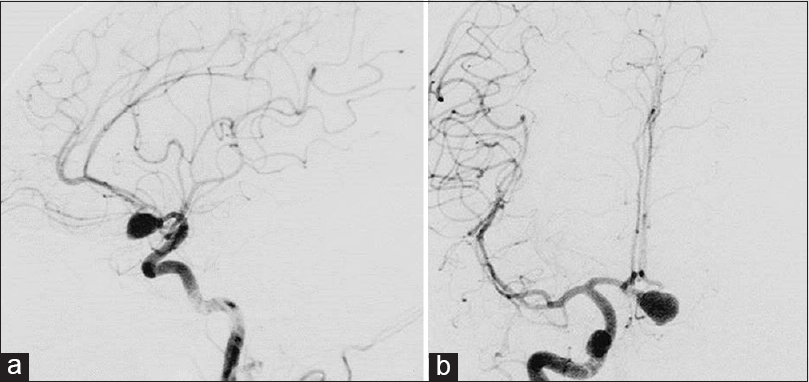
Three-dimensional computed tomography angiography on first admission (a) demonstrates distal anterior inferior cerebellar aneurysm (white arrow) as well as the absence of aneurysm of the right middle cerebral artery (MCA). Magnetic resonance angiography taken 23 months after initial treatment (b) shows de novo aneurysm of the right MCA (white arrow). One month later, the patient suffered subarachnoid hemorrhage in the right sylvian fissure by rupture of de novo aneurysm as shown by plain computed tomography on the second admission (c)
In terms of clinical outcomes, patients with ruptured regrown aneurysm showed the worst results at discharge, with an mRS score ≥4 in all cases (P = 0.003) [
DISCUSSION
The known risk factors for formation of a new aneurysm are female sex, young age, smoking, hypertension, and multiple aneurysms.[
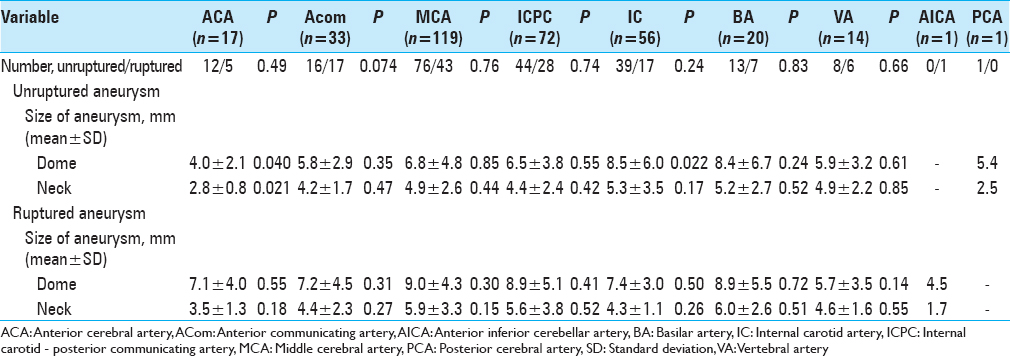
Our study agrees with previous studies that have revealed regrown aneurysms commonly develops when aneurysms are clipped incompletely at initial treatment, as well as obstructed with coils,[
To the best of our knowledge, the interval between initial treatment and retreatment, including for unruptured aneurysms, has not been reported. Some studies have shown a mean interval between initial SAH and retreatment with repeated SAH of 6.5–9.9 years.[
Long-term follow-up is also recommended. Our study showed that regrown aneurysm and de novo aneurysm rupture again in about 10 years from initial treatment. This result was consistent with results of previous surveys.[
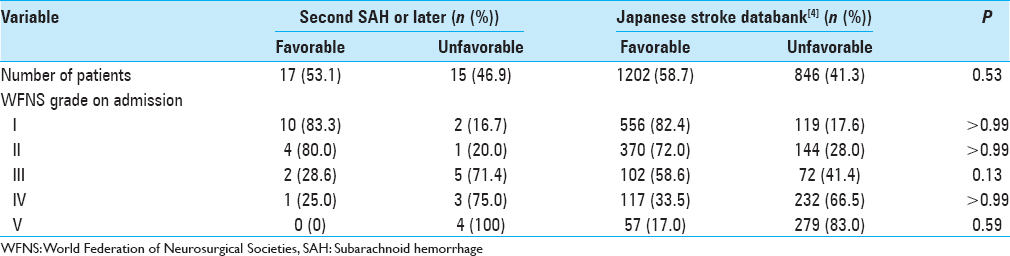
All patients who received retreatment for aneurysms that remained unruptured achieved good clinical outcomes in our study. For patients who received retreatment for aneurysm that had ruptured, clinical outcomes from our data consisting of second treatment or later showed a similar trend according to WFNS grade on admission as compared to the Japanese stroke databank in 2009 (which included any occasions of SAH) (Fisher's exact test; P = 0.14–0.99) [
This study had various limitations that need to be considered when interpreting the results.First, we do not know the total number of patients who received retreatment for intracranial aneurysms because this study only retrospectively identified patients who had been admitted to our hospital for aneurysm retreatment. Some patients might have been admitted to other hospitals for retreatment, and others might have died without precise diagnosis of SAH. Second, in this study, many known untreated aneurysms had been scheduled for retreatment in advance at the time of initial treatment, and therefore only a few aneurysms ruptured. In contrast, half of the retreatments for de novo aneurysms were for bleeding. This means that opportunities for appropriate diagnostic imaging and/or surgical or endovascular interventions may have been overlooked. The surgical and endovascular indications and intervals for follow-up diagnostic imaging also differ somewhat among surgeons, by patient age, and with patient ADL. As a result, the best solution on how to perform surgical or endovascular treatment in a timely manner for an individual patient remains unclear. Prospective trials using a large cohort after aneurysm treatment are needed to clarify these trends in our study. Third, this study only included Japanese patients. Risk of SAH is higher in Japanese populations, even though the incidence of unruptured aneurysm resembles that in Western populations.[
CONCLUSIONS
We need timely and long-standing follow-up regarding aneurysm status in patients who have once treated for aneurysm irrespective of treatment modality. For the first 2 years after initial treatment, regrown aneurysm at the originally treated site and known untreated aneurysms, in particular, show a high risk of rupture. Long-term follow-up is also needed to detect formation of de novo aneurysms from around 10 years after initial treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to thank Shotaro Yoshioka, M.D., Kentaro Hikichi, M.D., Keisho Sano, M.D., Yuka Sano, M.D., Shinya Kobayashi, M.D., Jun Tanabe, M.D., Hiroshi Saito, M.D., Masaki Maeda, M.D., Nobuharu Furuya, M.D., and Takuro Endo for their invaluable support in the acquisition of postoperative data and for intraoperative technical support.
References
1. David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999. 91: 396-401
2. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: The cerebral aneurysm rerupture after treatment (CARAT) study. Stroke. 2008. 39: 120-5
3. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001. 32: 485-91
4. Kakino S, Ogasawara K, Shibata T, Kobayashi S.editors. Japanese Stroke Databank 2009. Tokyo: Nakayama Shoten; 2009. p. 150-1
5. Nakagawa T, Hashi K, Kurokawa Y, Yamamura A. Family history of subarachnoid hemorrhage and the incidence of asymptomatic, unruptured cerebral aneurysms. J Neurosurg. 1999. 91: 391-5
6. Nakase H, Kamada Y, Aoki H, Goda K, Morimoto T, Sakaki T. Clinical study on recurrent intracranial aneurysms. Cerebrovasc Dis. 2000. 10: 255-60
7. Spiotta AM, Hui F, Schuette A, Moskowitz SI. Patterns of aneurysm recurrence after microsurgical clip obliteration. Neurosurgery. 2013. 72: 65-9
8. Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: Results of long-term follow-up angiography. Stroke. 2001. 32: 1191-4
9. Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of recurrent subarachnoid hemorrhage after complete obliteration of cerebral aneurysms. Stroke. 1998. 29: 2511-3
10. Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of subarachnoid hemorrhage after surgical treatment of unruptured cerebral aneurysms. Stroke. 1999. 30: 1181-4
11. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
12. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
13. Wermer MJ, Greebe P, Algra A, Rinkel GJ. Incidence of recurrent subarachnoid hemorrhage after clipping for ruptured intracranial aneurysms. Stroke. 2005. 36: 2394-9
14. Wermer MJ, Rinkel GJ, Greebe P, Albrecht KW, Dirven CM, Tulleken CA. Late recurrence of subarachnoid hemorrhage after treatment for ruptured aneurysms: Patient characteristics and outcomes. Neurosurgery. 2005. 56: 197-204
15. Wermer MJ, van der Schaaf IC, Velthuis BK, Algra A, Buskens E, Rinkel GJ. Follow-up screening after subarachnoid haemorrhage: Frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005. 128: 2421-9
16. Yamakawa H, Sakai N, Takenaka K, Yoshimura S, Andoh T, Yamada H. Clinical analysis of recurrent subarachnoid hemorrhage after neck clipping surgery. Neurol Med Chir (Tokyo). 1997. 37: 380-5