- Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa, Japan
Correspondence Address:
Kosei Yoshimura, Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa, Japan.
DOI:10.25259/SNI_620_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kosei Yoshimura, Sho Tsunoda, Masafumi Segawa, Mariko Kawashima, Tomohiro Inoue, Atsuya Akabane. Total removal of anaplastic meningioma infiltrating an artery by performing an A3–A3 side-to-side anastomosis. 13-Sep-2024;15:331
How to cite this URL: Kosei Yoshimura, Sho Tsunoda, Masafumi Segawa, Mariko Kawashima, Tomohiro Inoue, Atsuya Akabane. Total removal of anaplastic meningioma infiltrating an artery by performing an A3–A3 side-to-side anastomosis. 13-Sep-2024;15:331. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13092
Abstract
Background: Meningiomas are histologically benign tumors and generally have a good prognosis. However, some are classified as high-grade meningiomas due to their strong invasion of surrounding tissues and high postoperative recurrence rates, resulting in a poor prognosis. Postoperative radiotherapy is often administered for the most malignant anaplastic meningiomas; however, its contribution to improving prognosis and reducing recurrence rates in patients with residual tumors is limited.
Case Description: We present here a 48-year-old man with an anaplastic meningioma that recurred repeatedly and had invaded the right anterior cerebral artery (ACA) despite two postoperative radiotherapy sessions. Dissecting the tumor from the blood vessels was extremely difficult and would only have achieved a partial resection. However, we achieved complete resection by performing a pericallosal artery–pericallosal artery (A3–A3) side-to-side anastomosis and excising the infiltrated blood vessels along with the tumor en bloc. No neurological deficits or complications, such as cerebral infarction, were detected postoperatively.
Conclusion: Although reports of performing an A3–A3 side-to-side anastomosis to enable complete resection of tumors invading the ACA are extremely rare worldwide, this procedure should be recognized as a safe and effective treatment option when complete tumor resection is strongly desired, as in the present patient.
Keywords: A3–A3, Adjuvant radiotherapy, Anaplastic meningioma, Side-to-side anastomosis, Vascular infiltration
INTRODUCTION
High-grade meningiomas (World Health Organization [WHO] Grade II or III) are prone to invading surrounding normal tissues and are difficult to treat, often recurring even after a combination of surgical resection and postoperative radiotherapy.[
In the present case, a recurrent falcine meningioma had invaded the right pericallosal artery (A3). We, therefore, performed an A3–A3 side-to-side anastomosis distal to the tumor, enabling its total resection along with the infiltrated A3, resulting in a favorable outcome. Although reports of implementing A3–A3 side-to-side anastomosis for tumor resection are rare, it appears to be an effective treatment option when the tumor has invaded the anterior cerebral artery (ACA), as in the present patient. We, here, report this procedure with an accompanying surgical video [
Video 1
CLINICAL PRESENTATION
While residing in the USA for work, the patient, a 48-year-old man, had been hospitalized urgently 51 months previously with a chief complaint of gradually progressive impairment of consciousness. A 60-mm diameter tumor-like lesion that was strongly compressing the left frontal lobe from the interhemispheric fissure had been found [
Figure 1:
(a) MRI performed before the first operation showing an approximately 60 mm tumor in the interhemispheric fissure of the anterior cerebral region, compressing the left frontal lobe. (b) MRI performed after the first operation showed that the enhanced region had been totally removed. (c) MRI performed 20 months after the first operation showed enlargement of the tumor recurrence in the corpus callosum. (d) MRI performed before the second operation showed a growing tumor infiltrating the corpus callosum and anterior cerebral artery.
Intraoperative findings
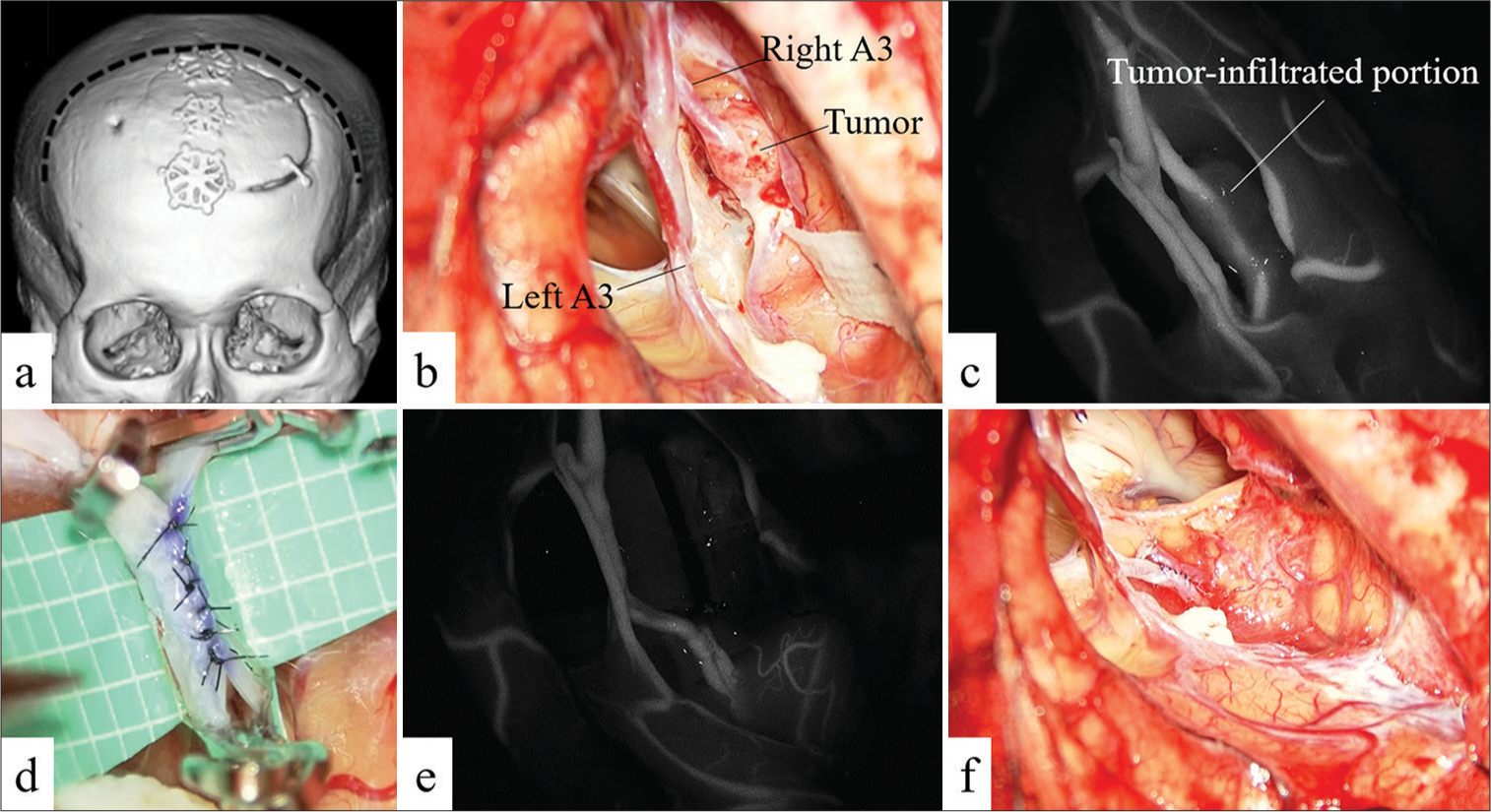
The patient was positioned supine with the head in the midline and secured with a Mayfield three-point fixation device. Somatosensory-evoked potential monitoring was prepared before surgery. The whole procedure was performed through a bicoronal skin incision with a large left frontal craniotomy extending across the midline [
Figure 2:
(a) Preoperative CT scan showing frontal craniotomy (previous bicoronal skin incision is shown as a dotted line). (b) Intraoperative photograph showing the right A3(Rt A3) is infiltrated by the tumor. (c) Indocyanine green (ICG) videography showing the tumor infiltrating a portion of A3. (d) Intraoperative photograph showing that an A3–A3 side-to-side anastomosis has been performed. (e) ICG videography showed the patency of the bypass. (f) Intraoperative photograph showing the tumor has been completely resected.
Initially, we attempted to separate the tumor from the right A3 but had to abandon this plan when we found that the tumor had infiltrated beyond the vascular adventitia and our dissection had inadvertently damaged this artery. The damaged section was trapped and sutured with 9-0 nylon, and hemostasis was achieved. Having determined that separating the tumor from the right A3 was not feasible, we decided to resect the tumor and infiltrated the A3 segment en bloc. In addition, since Doppler sonography showed that temporary clamping of the distal right A3 resulted in only a weak backflow, we decided that it was necessary to augment flow to the right ACA territory. The walls of both distal A3 arteries were free of atherosclerosis, making an A3–A3 bypass feasible.
We planned an A3–A3 side-to-side anastomosis distal to the site of tumor infiltration, where both A3 arteries run parallel. We sutured the posterior side of the anastomosis continuously and the anterior side with interrupted knots [
Post-operative course
Postoperative MRI showed that there were no complications, such as cerebral infarction [
DISCUSSION
Most meningiomas are benign and slow-growing; however, approximately 20–25% are classified as atypical meningiomas (WHO Grade II) and 1–3% as anaplastic meningiomas (WHO Grade III). The latter often grow rapidly, leading to poor outcomes.[
In the present case, despite undergoing surgery, external radiotherapy, and two rounds of Gamma Knife therapy for repeated recurrences of anaplastic meningioma, local control was not achieved, prompting a decision to pursue surgical total resection despite the identification of tumor invasion of the right A3. Partial resection without excising the infiltrated portion followed by postoperative radiotherapy was considered; however, it was evident that partial resection would be palliative in this case, given the history of multiple recurrences.
High-grade meningiomas sometimes invade the brain and surrounding tissues. When infiltrating an artery, the tumor first attacks the adventitia, its growth gradually resulting in the disappearance of the adventitia. The tumor then invades the media and elastic lamina and finally changes the morphology of the blood vessel such that there is no clear cleavage plane between the tumor and infiltrated area, making complete separation at the site of invasion difficult.[
In our patient, the tumor was very firmly attached to the arterial wall, and our attempts to remove it were unsuccessful because we were unable to find a clear plane for separation. Thus, it appeared that the only procedure that would achieve complete resection of the tumor at the site of vessel invasion was to remove the entire infiltrated blood vessel after creating an A3–A3 side-to-side anastomosis.
Revascularization to the ACA system can be roughly divided into two types, namely, intracranial–intracranial bypass such as A3–A3 side-to-side anastomosis (also known as in situ bypass) and revascularization from extracranial to intracranial arteries (extracranial–intracranial bypass). In the present patient, we also considered one of the latter: superficial temporal artery (STA)–A3 bypass using a free STA graft or an STA–radial artery graft–A3 bonnet bypass, the usefulness of which we previously reported.[
A review of published reports on implementing A3–A3 side-to-side anastomosis revealed very few reports globally,[
CONCLUSION
When a malignant meningioma has invaded a blood vessel, the high recurrence rate of these tumors mandates considering removing the tumor along with the invaded blood vessel. When there are no effective collateral channels to the area of the excised blood vessel, revascularization must be performed. A3–A3 side-to-side anastomosis should be considered a good option for meningiomas infiltrating the ACA.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Video available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
We thank Dr. Trish Reynolds, MBBS, FRACP, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
1. Dunn GP, Gerrard JL, Jho DH, Ogilvy CS. Surgical treatment of a large fusiform distal anterior cerebral artery aneurysm with in situ end-to-side A3-A3 bypass graft and aneurysm trapping: Case report and review of the literature. Neurosurgery. 2011. 68: E587-91
2. Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS. Malignant meningioma: An indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998. 37: 177-88
3. Frisoli FA, Srinivasan VM, Catapano JS, Lawton MT. Resection of a recurrent, irradiated hemangiopericytoma with A3-A3 anterior cerebral artery bypass: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2022. 22: e280
4. Holleczek B, Zampella D, Urbschat S, Sahm F, von Deimling A, Oertel J. Incidence, mortality and outcome of meningiomas: A population-based study from Germany. Cancer Epidemiol. 2019. 62: 101562
5. Labib MA, Gandhi S, Cavallo C, Nisson PL, Mooney MA, Catapano JS. Anterior cerebral artery bypass for complex aneurysms: Advances in intracranial-intracranial bypass techniques. World Neurosurg. 2020. 141: e42-54
6. Qin C, Huang M, Pan Y, Li Y, Long W, Liu Q. Brain-invasive meningiomas: Molecular mechanisms and potential therapeutic options. Brain Tumor Pathol. 2021. 38: 156-72
7. Tsunoda S, Inoue T, Segawa M, Akabane A. One-stage revascularization to the ipsilateral middle cerebral artery and contralateral anterior cerebral artery territories. World Neurosurg. 2022. 164: 128-34
8. Tsunoda S, Inoue T, Segawa M, Okubo S, Akabane A. Revascularization to the ACA: Effectiveness and variation of the STA-RAG-A3 bonnet bypass. Acta Neurochir. 2021. 163: 3483-93
9. Wang JZ, Nassiri F, Aldape K, Zadeh G. Introduction: Ongoing clinical challenges in the management of meningiomas and future directions. Neurooncol Adv. 2023. 5: i1-4








Kyung Gi Cho
Posted September 17, 2024, 6:43 pm
It’s very interesting paper. I would like to see your surgical video.