- Department of Neurosurgery, Helsinki University Hospital, Helsinki, Finland
- Department of Pediatric Neurology and Neurosurgery, North-Western State Medical University, Saint-Petersburg, Russia
Correspondence Address:
Juha Hernesniemi
Department of Neurosurgery, Helsinki University Hospital, Helsinki, Finland
DOI:10.4103/2152-7806.189298
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kozyrev DA, Jahromi BR, Hernesniemi J. Total temporary occlusion of blood flow for several hours to treat a giant deep arteriovenous malformation: A series of multiple operations to save a young life. Surg Neurol Int 26-Aug-2016;7:79
How to cite this URL: Kozyrev DA, Jahromi BR, Hernesniemi J. Total temporary occlusion of blood flow for several hours to treat a giant deep arteriovenous malformation: A series of multiple operations to save a young life. Surg Neurol Int 26-Aug-2016;7:79. Available from: http://surgicalneurologyint.com/surgicalint_articles/total-temporary-occlusion-blood-flow-several-hours-treat-giant-deep-arteriovenous-malformation-series-multiple-operations-save-young-life/
Abstract
Background:The treatment of giant deep arteriovenous malformations (AVMs) remains challenging.
Case Description:We report a case of giant deep AVM diagnosed in a 9-year-old girl, for whom the AVM rupture occurred 9 years later. At the age of 9, the girl developed mild left hemiparesis. Magnetic resonance imaging revealed a giant deep AVM. The patient underwent one course of stereotactic radiotherapy followed by serial imaging. At the age of 18, we admitted her to our department with left hemiparesis and a loss of consciousness. Computed tomography showed intracerebral hemorrhage related to AVM. The treatment process proved challenging, with recurrent intracerebral hemorrhages. During the second operation, we used total temporary occlusion for almost 4 hours. Eventually, after 4 rounds of embolizations, 4 microsurgical operations, and a month-and-a-half after admission, AVM was successfully occluded. Five years after this treatment, the patient regained the ability to walk without assistance, although a moderate disability with visual changes remained (Modified Rankin Scale score 3).
Conclusion:This case illustrates that the cumulative risk of rupture of a high-grade AVM in young patients is evident, while treatment may prove successful with satisfactory results.
Keywords: Arteriovenous malformation, deep AVM, intracerebral hemorrhage, microneurosurgery
BACKGROUND
Intracranial arteriovenous malformation (AVM) is a tangle of abnormal blood vessels with no intermediary capillary system. A vast majority of all lesions are thought to have a congenital nature, however, at present, some authors report noncongenital cases.[
AVM is considered to be giant when nidus is 6 cm or more in maximum diameter.[
Ruptured AVM associated with a large intraparenchymal hematoma needs urgent surgical intervention. In such a case, surgery is a life-saving procedure, and the main aim is removal of hematoma. Resection of AVM depends upon patient's condition, the complexity of the AVM, and surgeon's and department's experience. We present one of the cases with unique staged multidisciplinary treatment. Outcomes were assessed with the Modified Rankin Scale (mRS) score after the operation and at late follow-up.
CASE DESCRIPTION
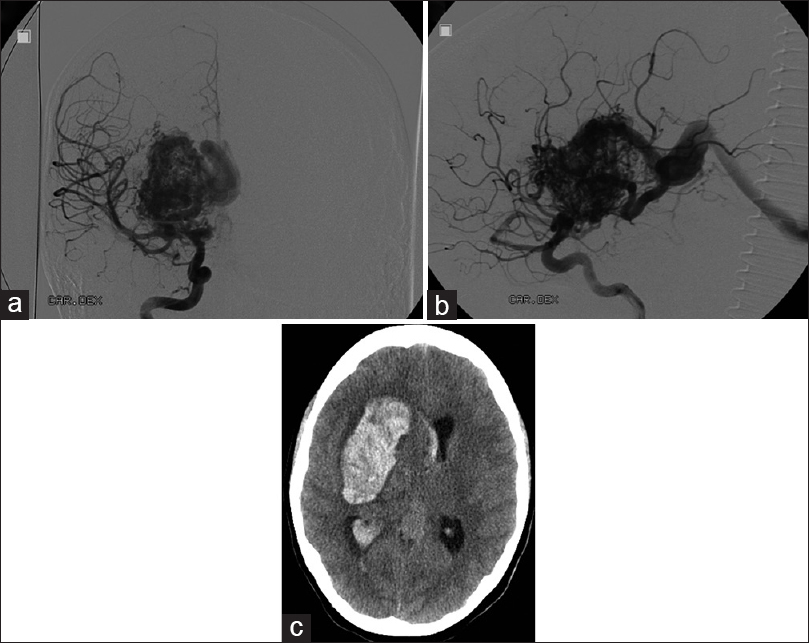
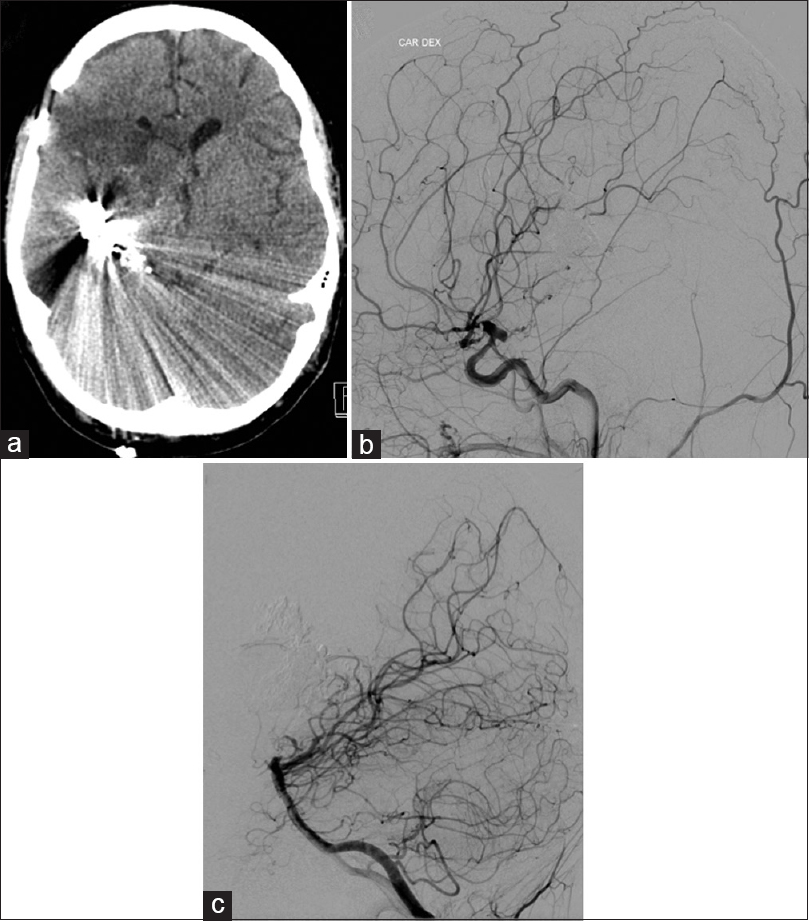
The 9-year-old otherwise healthy girl presented with minimal physical clumsiness and progressive mild left hemiparesis. Magnetic resonance imaging (MRI) revealed a giant deep AVM. This lesion was considered a grade-V AVM according to Spetzler–Martin scale. Surgical treatment of the AVM was declined as it was too risky. Radiosurgical treatment was instituted due to the size and location of the AVM. She received one course of radiotherapy (20 Gr) resulting in a slight reduction in the size of the AVM. Last follow-up digital subtraction angiography (DSA) at the age of 12 showed no change in AVM [Figure
The decision on the surgical treatment of the AVM was made with a help of endovascular embolization. Onyx embolization was admitted with minor success. Treatment continued with microsurgery. The patient was taken to the operative theater for the removal of intracerebral hematoma and resection of the AVM. During the evacuation of hematoma major intraoperative bleeding (1 L) occurred from Onyx embolized intranidal aneurysm. Bleeding was effectively held by emergency clipping of some feeders originating from the middle cerebral artery (MCA). Resection of the AVM could not be performed because of the unstable medical conditions of the patient.
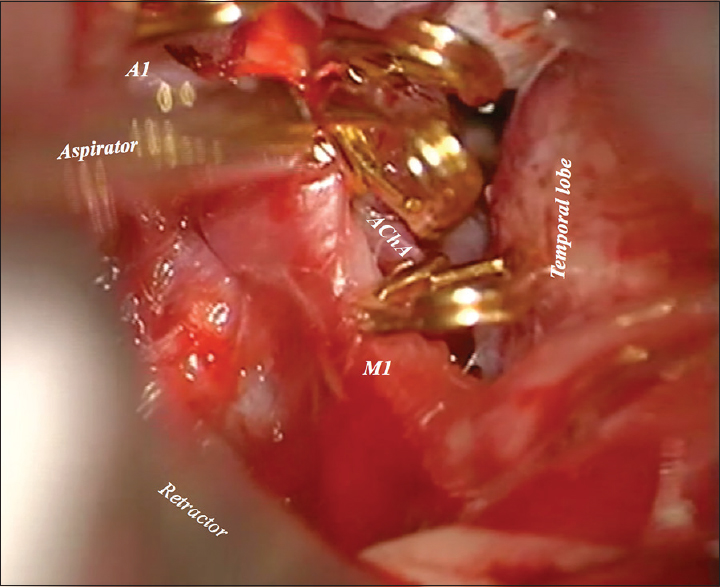
Postoperatively, the patient clinical condition was the same neurologically as during admission. However, 3 days after the first operation, the patient's condition deteriorated suggesting intracranial hypertension. The patient underwent a placement of external drainage catheter with the intracranial pressure monitor. Intracranial hypertension was eliminated. Further treatment continued with a new round of endovascular modality. A new attempt of Onyx embolization through the posterior cerebral artery (PCA) was made. On the same day, the patient underwent a second microsurgical operation using the same craniotomy. We used total temporary clipping under mild hypothermia (33°C) for almost 4 hours; P2 segment of PCA, A1 segment of anterior cerebral artery, anterior choroidal artery (AChA), and the supraclinoid segment of internal carotid artery (ICA) [
Figure 3
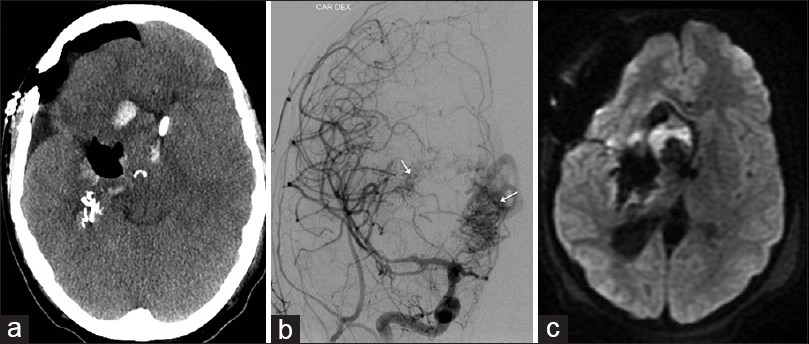
Postoperative images after the 2nd operation: axial СT scan (a) air instead of main part of arteriovenous malformation (AVM); digital subtraction angiography (posterioranterior view) (b) medial and later residual parts of AVM (arrows); axial diffusion weighted imaging scan (c); only focal ischemia adjacent to remnant part in basal ganglia
Three weeks after the third operation, shunt for cerebrospinal fluid diversion was inserted, and the patient remained conscious with left hemiparesis. The patient was transported to the department of neurology for rehabilitation. However, few days later, new minor bleeding occurred, adjacent to the residual part of the AVM. Two embolizations were performed through MCA and PCA. Residual part filling via MCA was completely occluded, however, small residual filling via PCA remained [Figure
Figure 4
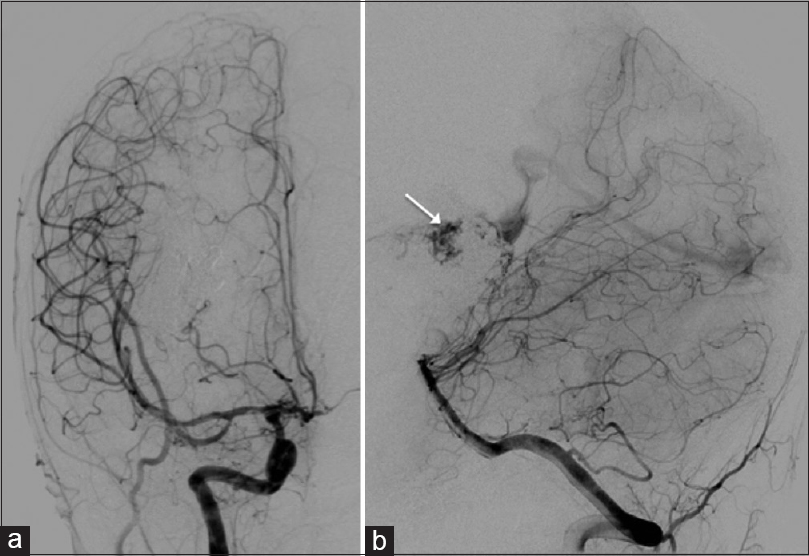
Postoperative angiographic images after the 4th embolization: Digital subtraction angiography (DSA) (posterioranterior view) (a) no filling of arteriovenous malformation through middle cerebral artery; DSA (lateral view) (b) small residual part filling through branch of posterior cerebral artery (arrow)
Shunt for cerebrospinal fluid diversion was removed. Ten days later, the patient underwent the last microsurgical operation. Eventually, AVM was completely occluded [Figure
DISCUSSION
Cerebral giant deep arteriovenous malformations are complex lesions with highly challenging treatments. In addition, giant AVMs and deep-seated AVMs have consistent risk factors for future bleeding.[
Although it is probable that deep AVMs carry a higher hemorrhage risk, it is clear that these AVMs also carry a higher surgical treatment risk.[
In general, for deep AVMs without safe surgical access, initial treatment of choice should be radiosurgery and/or endovascular intervention. Nevertheless, radiosurgery carries a relatively lower long-term obliteration rate and has a high risk of radiation-related complications compared with AVMs in other locations. Potts et al. found that 18% of patients initially treated only with radiosurgery of deep AVMs have post-treatment hemorrhage and the annual rate is 3.9%.[
As illustrated in this report, specific clinical situations may compel surgical intervention,[
CONCLUSION
This case demonstrates a complex problem that encountered during treatment of giant deep AVM. Such kind of AVMs should have staged multimodality treatment and final aim is complete occlusion. This eliminates the risk of future hemorrhages that is crucial for young individuals with a long life expectancy. Even a small remnant part of an AVM can cause hemorrhage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bulsara KR, Alexander MJ, Villavicencio AT, Graffagnino C. De novo cerebral arteriovenous malformation: Case report. Neurosurgery. 2002. 50: 1137-40
2. Chang SD, Marcellus ML, Marks MP, Levy RP, Do HM, Steinberg GK. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003. 53: 1-11
3. Drake CG. Cerebral arteriovenous malformations: Considerations for and experience with surgical treatment in 166 cases. Clini Neurosurg. 1979. 26: 145-208
4. Duckworth EA, Gross B, Batjer HH. Thalamic and basal ganglia arteriovenous malformations: Redefining “inoperable”. Neurosurgery. 2008. 63: S63-7
5. Fleetwood IG, Marcellus ML, Levy RP, Marks MP, Steinberg GK. Deep arteriovenous malformations of the basal ganglia and thalamus: Natural history. J Neurosurg. 2003. 98: 747-50
6. Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: A long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008. 63: 823-9
7. Kilbourn KJ, Spiegel G, Killory BD, Kureshi I. Case report of a de novo brainstem arteriovenous malformation in an 18-year-old male and review of the literature. Neurosurg Rev. 2014. 37: 685-91
8. Laakso A, Dashti R, Juvela S, Isarakul P, Niemela M, Hernesniemi J. Risk of hemorrhage in patients with untreated Spetzler-Martin grade IV and V arteriovenous malformations: A long-term follow-up study in 63 patients. Neurosurgery. 2011. 68: 372-7
9. Laakso A, Dashti R, Juvela S, Niemela M, Hernesniemi J. Natural history of arteriovenous malformations: Presentation, risk of hemorrhage and mortality. Acta Neurochir Suppl. 2010. 107: 65-9
10. Mahajan A, Manchandia TC, Gould G, Bulsara KR. De novo arteriovenous malformations: Case report and review of the literature. Neurosurg Rev. 2010. 33: 115-9
11. Nataraj A, Mohamed MB, Gholkar A, Vivar R, Watkins L, Aspoas R. Multimodality treatment of cerebral arteriovenous malformations. World Neurosurg. 2014. 82: 149-59
12. Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: A 24-year follow-up assessment. J Neurosurg. 1990. 73: 387-91
13. Potts MB, Jahangiri A, Jen M, Sneed PK, McDermott MW, Gupta N. Deep arteriovenous malformations in the basal ganglia, thalamus, and insula: Multimodality management, patient selection, and results. World Neurosurg. 2014. 82: 386-94
14. Ujiie H, Higa T, Hayashi M, Tamano Y, Muragaki Y, Hori T. Surgical management of Spetzler-Martin grade V AVM. J Clin Neurosci. 2002. 9: 22-5
15. White JA, Batjer HH. Management of deep arteriovenous malformations. World Neurosurg. 2015. 83: 339-40