- Department of Neurosurgery, Tokushima Red Cross Hospital, Komatsushima, Tokushima,
- Department of Neurosurgery, Kawasaki Medical School, Kurashiki, Okayama,
- Department of Neurosurgery, Tokushima University, Tokushima,
- Department of Neurology, Tokushima Red Cross Hospital, Komatsushima, Tokushima,
- Department of Neurosurgery, Shikoku Medical Center for Children and Adults, Zentsuji, Kagawa, Japan.
Correspondence Address:
Masaaki Korai, Department of Neurosurgery, Tokushima Red Cross Hospital, Komatsushima, Tokushima, Japan.
DOI:10.25259/SNI_215_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masaaki Korai1, Noriya Enomoto1, Koichi Satoh1, Shunji Matsubara2, Yasuhisa Kanematsu3, Tadashi Yamaguchi1, Mami Hanaoka1, Hitoshi Niki4, Kazuhito Matsuzaki1, Koji Bando3, Hirotaka Hagino5, Yasushi Takagi3. Transarterial embolization for convexity dural arteriovenous fistula with or without pial arterial supply: A report of four patients. 05-Aug-2022;13:340
How to cite this URL: Masaaki Korai1, Noriya Enomoto1, Koichi Satoh1, Shunji Matsubara2, Yasuhisa Kanematsu3, Tadashi Yamaguchi1, Mami Hanaoka1, Hitoshi Niki4, Kazuhito Matsuzaki1, Koji Bando3, Hirotaka Hagino5, Yasushi Takagi3. Transarterial embolization for convexity dural arteriovenous fistula with or without pial arterial supply: A report of four patients. 05-Aug-2022;13:340. Available from: https://surgicalneurologyint.com/surgicalint-articles/11766/
Abstract
Background: Convexity dural arteriovenous fistulae (dAVF) usually reflux into cortical veins without involving the venous sinuses. Although direct drainage ligation is curative, transarterial embolization (TAE) may be an alternative treatment.
Case Description: Between September 2018 and January 2021, we encountered four patients with convexity dAVFs. They were three males and one female; their age ranged from 36 to 73 years. The initial symptom was headache (n = 1) or seizure (n = 2); one patient was asymptomatic. In all patients, the feeders were external carotid arteries with drainage into the cortical veins; in two patients, there was pial arterial supply from the middle cerebral artery. All patients were successfully treated by TAE alone using either Onyx or N-butyl cyanoacrylate embolization. Two patients required two sessions. All dAVFs were completely occluded and follow-up MRI or angiograms confirmed no recurrence.
Conclusion: Our small series suggests that TAE with a liquid embolic material is an appropriate first-line treatment in patients with convexity dAVFs with or without pial arterial supply.
Keywords: Convexity, Dural arteriovenous fistula, Pial artery, Transarterial embolization
INTRODUCTION
Dural arteriovenous fistulae (dAVFs) are the result of anomalous connections between the meningeal arteries and dural veins.[
Direct surgery is a radical treatment for convexity dAVFs; however, endovascular treatment is another option. It is difficult to access dAVFs through their venous drainage route to the shunt point and there is also a risk of venous rupture. Therefore, transarterial embolization (TAE) can be selected instead of transvenous embolization. Our literature search found no reports of convexity dAVFs with pial arterial supply that were successfully treated by TAE alone.
Using Onyx (Medtronic, MN, USA) or N-butyl cyanoacrylate (NBCA) for TAE, we successfully treated four patients with convexity dAVFs; two patients presented with pial arterial supply. Here, we report their clinical features and their successful treatment with TAE alone.
CASE REPORTS
Representative case 1
A 63-year-old man scheduled for heart valve surgery underwent magnetic resonance imaging. A flow void in the left occipital lobe was detected incidentally [
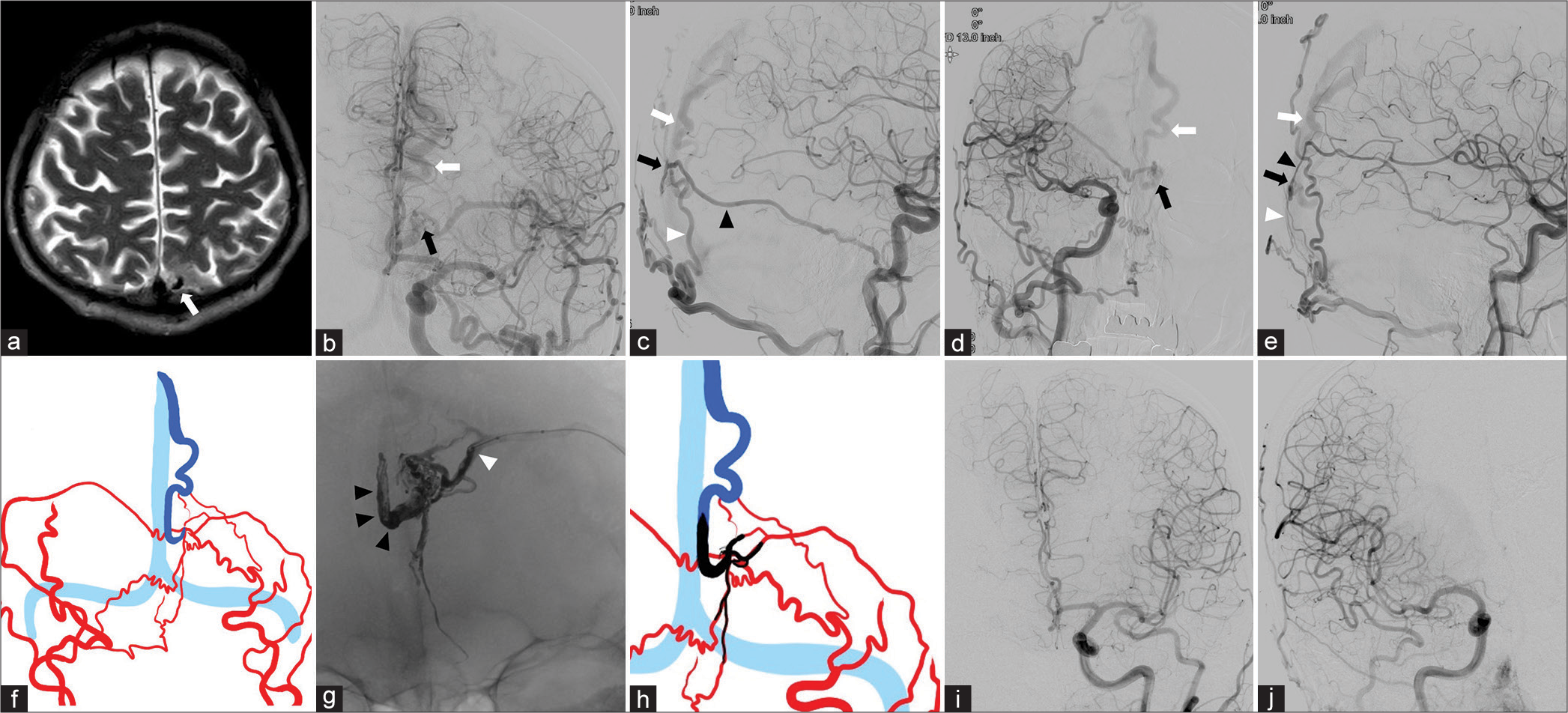
Figure 1:
Representative Case 1 (a) T2-weighted image shows flow void in the medial aspect of the left occipital lobe (white arrow). (b and c) The left common carotid angiograms (b: frontal view and c: lateral view) confirm a dural arteriovenous fistula (dAVF) fed by the mastoid branch of the left occipital artery (OA) (white arrowhead) and middle meningeal artery (MMA) (black arrowhead) on the left occipital convexity with cortical venous reflux (CVR, white arrow). The black arrow indicates the shunt site. (d and e) The right common carotid angiograms (d: frontal view and e: lateral view) reveal the dAVF (white arrow) supplied by the mastoid branch of the right OA (white arrowhead) and MMA (black arrowhead) with the drainage into the occipital cortical vein. The black arrows indicate the shunt site. (f) Schema of the angioarchitecture delineating the feeding arteries (red), CVR (blue), and sinus (light blue). (g) Postoperative magnified X-ray (frontal view) demonstrates that the CVR, the mastoid branch of the left OA, and the posterior branch of the MMA were filled with Onyx (black arrowhead). The white arrowhead indicates the injection point on the MMA (tip of the balloon catheter). (h) Schematic drawing of the Onyx embolization material (black), feedeing arteries (red), CVR (blue), and sinus (light blue). (i and j) Postoperative left (i) and right (j) common carotid injections (frontal view) identify complete disappearance of the dAVF.
With the patient under general anesthesia, we advanced a 6-Fr guiding catheter into the left external carotid artery through the femoral artery. A balloon catheter (Scepter XC; Microvention, CA, USA) was navigated to the distal portion of the posterior branch of the left MMA [
Representative case 2
A 42-year-old woman developed headache and vertigo. MRI identified flow voids and dilated cortical vessels in and around the left central sulcus [
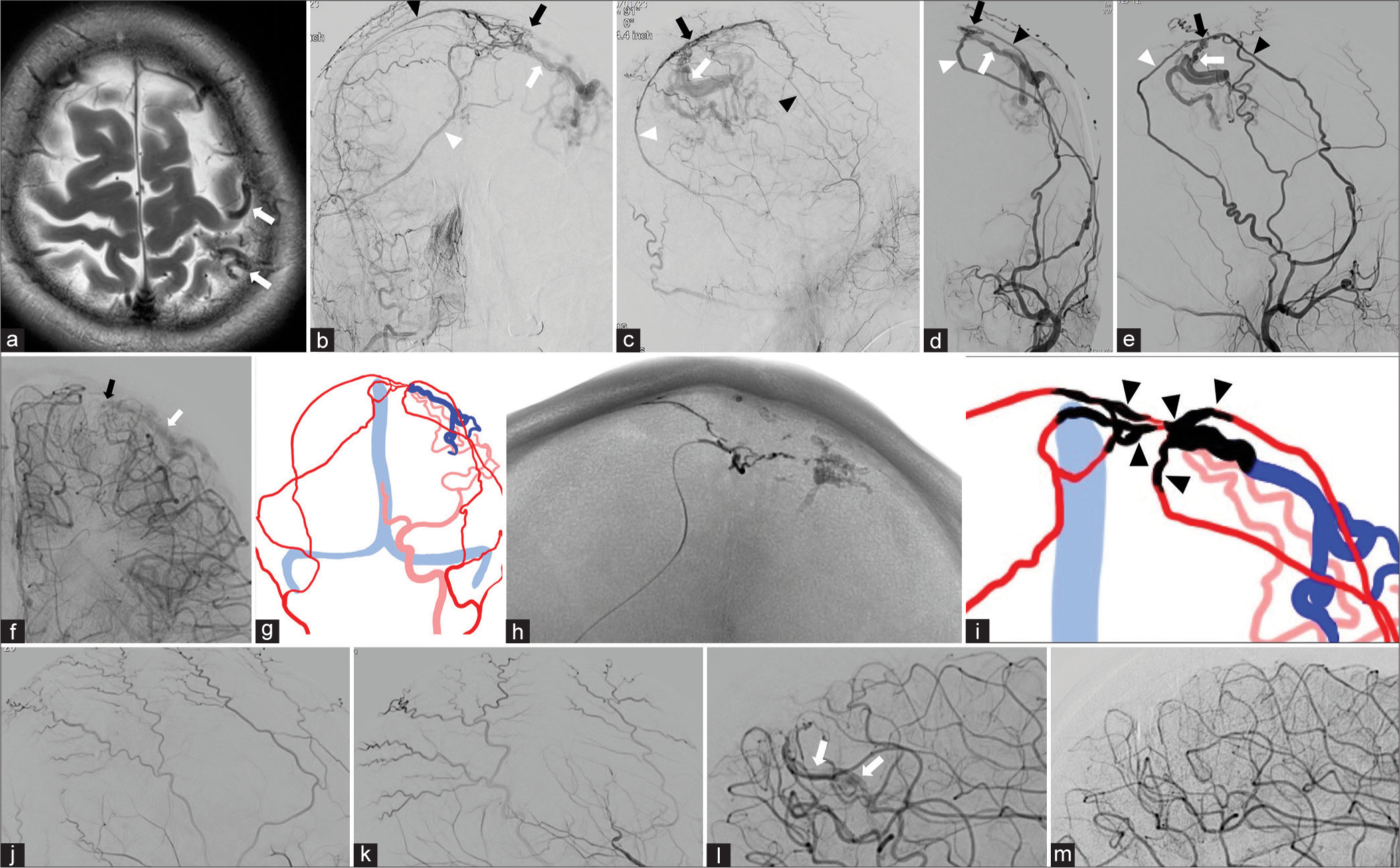
Figure 2:
Representative Case 2: (a) T2-weighted image shows dilated cortical vessels (white arrows) in and around the left central sulcus. (b and c) Selective right external carotid angiograms (b: frontal view and c: lateral view) identified a dural arteriovenous fistula (dAVF) on the left parietal convexity. It was fed from the right anterior (black arrowhead) and posterior (white arrowhead) branch of the middle meningeal artery (MMA). Drainage was into a dilated Rolandic vein (white arrow). Black arrows indicates the shunt site. (d and e) Selective left external angiograms (D: frontal view and E: lateral view) also reveal the arteriovenous shunt arising from the left anterior (black arrowhead) and posterior (white arrowhead) branch of the MMA with cortical venous reflux (CVR) (white arrows). The black arrows indicate the shunt site. (f) Selective left internal carotid angiogram (frontal view) demonstrates pial arterial supply from the left middle cerebral artery (MCA). Black and white arrows indicate the shunt site and CVR, respectively. (g) Schema of the angioarchitecture delineating the feeding arteries from the external carotid arteries (red), the pial arterial supply from the left MCA (pink), CVR (dark blue), and the sinus (light blue). (h) X-ray (frontal view) demonstrates CVR and the bilateral MMAs filled with NBCA. (i) Schematic drawing of NBCA distribution (black). Black arrowheads indicate the 5 NBCA injection sites. Red, pink, dark blue and light blue also indicate feeding arteries, the pial arterial supply, CVR and the sinus respectively. (j and k) Selective right (j: lateral view) and left external (k: lateral view) carotid angiograms confirming complete obliteration of the dAVF immediately after the second TAE session. (l and m) Selective left internal carotid angiograms (lateral views) demonstrate a slight residual shunt flow (white arrows) from the left MCA just after the second session (l), and complete disappearance of the shunt 6 months after the procedures (m).
At a second TAE session, performed 1 month later, we injected NBCA (25% concentration) through the posterior branch of the right and the anterior branch of the left MMA [
RESULTS
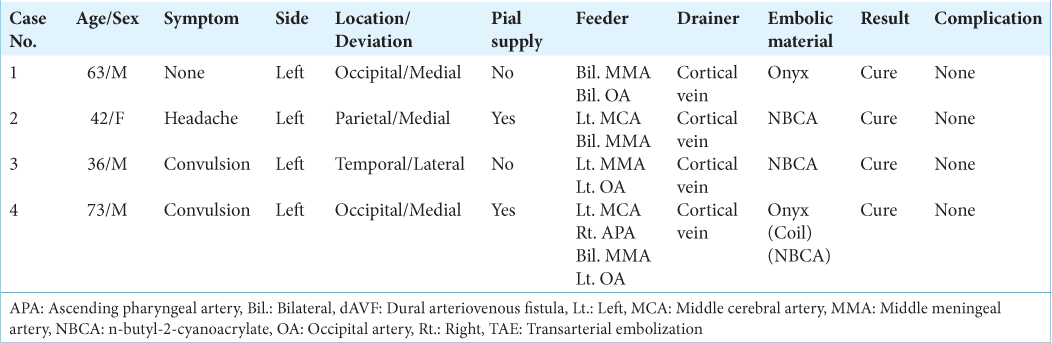
The etiology of their dAVFs was unknown in all four patients. They had no history of sinus or venous thrombosis, no blood coagulation abnormalities, no head trauma, and none had undergone craniotomy. As shown in
DISCUSSION
Since convexity dAVFs are non-sinusal, they often exhibit CVR. Intracranial hemorrhage was elicited by 43% of convexity dAVFs reported by Kobayashi et al.[
During TAE for convexity dAVFs, the fistula site must be penetrated, therefore, the microcatheter must be advanced as close as possible to that site. In Cases 2 and 3, the microcatheter was a DeFrictor; the outer diameter of the tip was 1.3-Fr. The DeFrictor microcatheter features a flow-direct and a guidewire catheter. It provides excellent flexibility, trackability, and extremely high distal reachability. Because we were able to advance the DeFrictor very close to the fistula site in Cases 2 and 3, we were able to deliver NCBA to the cortical vein.
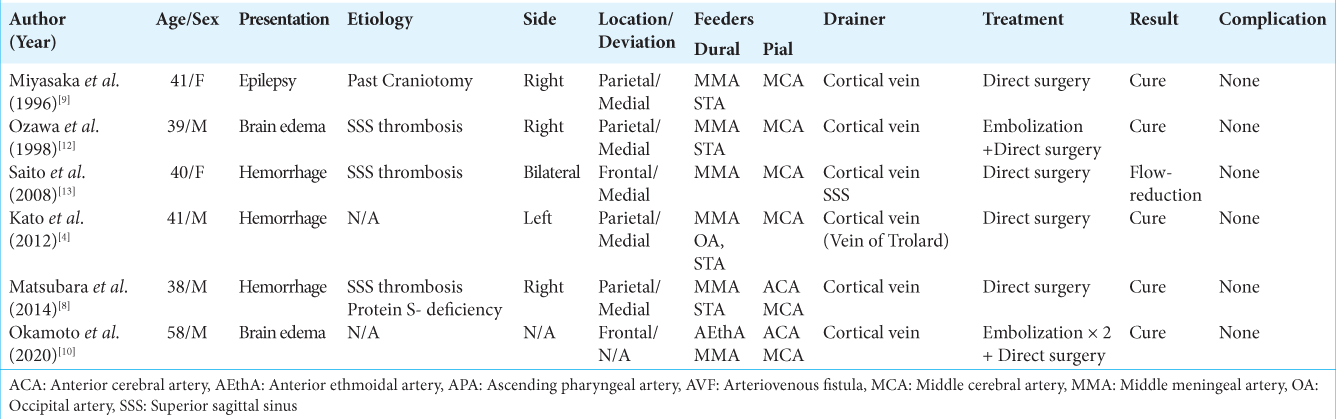
Others[
Although both injected Onyx and NBCA may flow into the pial artery, we think that Onyx is more likely to result in complete obliteration because the liquid material tends to move toward the venous side that is under lower pressure before filling the pial arterial feeders. Consequently, before embolization, it is important to identify the appropriate distance from the fistula to the Onyx injection site and the delivery of Onyx must be stopped when the liquid material reaches the predefined position. In cases, where the microcatheter can be advanced to be close to the fistulous site, NBCA may be an alternative embolization material. When the dAVF with pial arterial supply is adjacent to the eloquent area such as the motor cortex, direct surgery may be indicated to avoid ischemic complications.
CONCLUSION
Open surgery can be chosen as a radical therapy to treat dAVFs. However, our small case series suggests that TAE can be a less invasive and curative treatment in patients with convexity dAVFs, even in the presence of pial arterial supply. Embolization with Onyx may be more advantageous in terms of the cure rate, although the delivery of NBCA is possible in cases where a very thin microcatheter can be advanced very close to the shunt site.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformation and implications of treatment. J Neurosurg. 1995. 82: 166-79
2. Brinjikji W, Cloft HJ, Lanzino G. Clinical, angiographic, and treatment characteristics of cranial dural arteriovenous fistulas with pial arterial supply. J Neurointerv Surg. 2021. 13: 331-5
3. Hetts SW, Yen A, Cooke DL, Nelson J, Jolivalt P, Banaga J. Pial artery supply as an anatomic risk factor for ischemic stroke in the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2017. 38: 2315-20
4. Kato N, Tanaka T, Suzuki Y, Sakamoto H, Arai T, Hasegawa Y. Multistage indocyanine green videoangiography for the convexity dural arteriovenous fistula with angiographically occult pial fistula. J Stroke Cerebrovasc Dis. 2012. 21: 918.e1-e5
5. Kobayashi E, Wakamatsu S, Tominaga S. A case of dural arteriovenous malformation on the convexity adjacent to the superior sagittal sinus. No Shinkei Geka. 1994. 22: 643-8
6. Kuwayama N, Kubo M, Endo S, Sakai N. Present status in the treatment of dural arteriovenous fistulas in Japan. Jpn J Neurosurg (Tokyo). 2011. 20: 12-9
7. Lasjaunias P, Chui M, ter Brugge K, Tolia A, Hurth M, Bernstein M. Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg. 1986. 64: 724-30
8. Matsubara S, Satoh K, Satomi J, Shigekiyo T, Kinouchi T, Miyake H. Acquired pial and dural arteriovenous fistulae following superior sagittal sinus thrombosis in patients with protein S deficiency: A report of two cases. Neurol Med Chir (Tokyo). 2014. 54: 245-52
9. Miyasaka Y, Kurata A, Saegusa H, Yuzawa I, Utsuki S, Ohwada T. Dural-pial arteriovenous malformation with unusual venous drainage. Neurol Med Chir (Tokyo). 1996. 36: 91-5
10. Okamoto M, Sugiyama T, Nakayama N, Ushikoshi S, Kazumata K, Osanai T. Microsurgical findings of pial arterial feeders in intracranial dural arteriovenous fistulae: A case series. Oper Neurosurg (Hagerstown). 2020. 19: 691-700
11. Osada T, Krings T. Intracranial dural arteriovenous fistulas with pial arterial supply. Neurosurgery. 2019. 84: 104-15
12. Ozawa T, Miyasaka Y, Tanaka R, Kurata A, Fujii K. Dural-pial arteriovenous malformation after sinus thrombosis. Stroke. 1998. 29: 1721-4
13. Saito A, Sugawara T, Mikawa S, Akamatsu Y, Saito H, Seki H. A case of multiple pial arteriovenous fistulas associated with dural arteriovenous fistula. J Neurosurg. 2008. 109: 1103-7
14. van Dijk JM, ter Brugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002. 33: 1233-6