- Department of Neurosurgery, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan
Correspondence Address:
Takahiro Ota, Department of Neurosurgery, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
DOI:10.25259/SNI_1070_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takahiro Ota. Transarterial embolization of convexity meningioma via the meningolacrimal artery through the ophthalmic artery: A case report with embryological insights. 04-Apr-2025;16:122
How to cite this URL: Takahiro Ota. Transarterial embolization of convexity meningioma via the meningolacrimal artery through the ophthalmic artery: A case report with embryological insights. 04-Apr-2025;16:122. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13484
Abstract
BackgroundThe presence of dangerous anastomoses between the ophthalmic artery (OphA) and the middle meningeal artery has the potential risk of causing visual function complications during transarterial embolization.
Case DescriptionThe present case was a 69-year-old male with convexity meningioma. The main trunk of the middle meningeal artery was coagulation-cut at the level of the foramen spinosum because a combined petrosal approach was applied 22 years earlier at the time of craniotomy. Preoperative digital subtraction angiography showed the characteristic dual feeding pattern. The tumor was supplied with blood flow from the meningolacrimal artery, which is a branch of the OphA, and the anteromedial branch of the inferolateral trunk, which is a branch of the internal carotid artery (ICA), through the middle meningeal artery anastomoses on the dura mater. We successfully embolized the feeder transarterially through the meningolacrimal artery through the OphA.
ConclusionThe presence of the dual feeding pattern from both the OphA and the inferolateral trunk of the ICA is rare, but embryological considerations gave us an important clue to understand the development of the angioarchitecture and evaluate treatment risks.
Keywords: Anastomosis, Embryology, Meningolacrimal artery, Ophthalmic artery
INTRODUCTION
The middle meningeal artery (MMA) is the main arterial supplier of the convexity dura mater, and it is well known to have potential anastomoses with the ophthalmic artery (OphA) during the developmental process. In particular, the presence of the superior orbital fissure (SOF)-mediated recurrent meningeal artery and the lacrimal foramen-mediated meningolacrimal artery has been noted to cause visual function complications during transarterial embolization from MMA potentially.
However, reports of transarterial embolization through the OphA, including meningiomas and retinoblastomas, are increasing. To prevent embolic complications, understanding the dangerous extracranial-intracranial anastomoses around the orbit is essential, as the branches of MMA on the dura mater have abundant anastomoses. Here, we report a case in which the presence of these anastomoses required further attention.
CASE PRESENTATION
A 69-year-old male presented after a family member noticed abnormal behavior several months earlier. He subsequently developed a walking disorder and was referred to our department with a suspected meningioma. Clinical evaluation revealed clear cognitive results and left hemiparesis at the manual muscle testing (MMT) 4/5 level. He had dysarthria and right-sided facial sensory numbness.
Twenty-two years ago, a right trigeminal schwannoma was removed through a right combined petrosal approach.
Magnetic resonance imaging showed a T1-gadolinium (T1Gd) homogenous enhanced lesion, 45 x 40 x 45 mm in size, which was supposed to be a convexity meningioma [
Figure 1:
Preoperative magnetic resonance imaging (MRI) and digital subtraction angiography. (a) T2-weighted image. Right frontal convexity meningioma with extensive peritumoral edema. (b and c) T1 Gd-enhanced MRI showing an irregularly shaped, homogeneously enhanced tumor based on the frontal convexity of the dura mater. 45 × 40 mm × 45 mm, respectively. (d and e) Right internal carotid angiography revealed the feeder from the ophthalmic artery (white arrow). (f) Right external carotid angiography showed no middle meningeal artery.
Digital subtraction angiography (DSA) was performed preoperatively. Right external carotid angiography revealed no MMA due to a coagulation cut in the previous operation [
Figure 2:
Preoperative digital subtraction angiography images. (a) 3D-rotational angiography (right ICA), lateral view. The main feeder to the tumor was the anterior convexity branch of the MMA, which was supplied from both the MLA and the anteromedial branch of the ILT. The CRA was clearly shown to originate from the OphA. (b-e) Cone-beam CT (right ICA) reveals the origin of the ILT and the MLA passing through the meningolacrimal foramen. ICA: Internal carotid artery, MMA: Middle meningeal artery, MLA: Meningolacrimal artery, ILT: Inferior lateral trunk, CRA: Central retinal artery, OphA: Ophthalmic artery, CT: Computed tomography.
Procedure
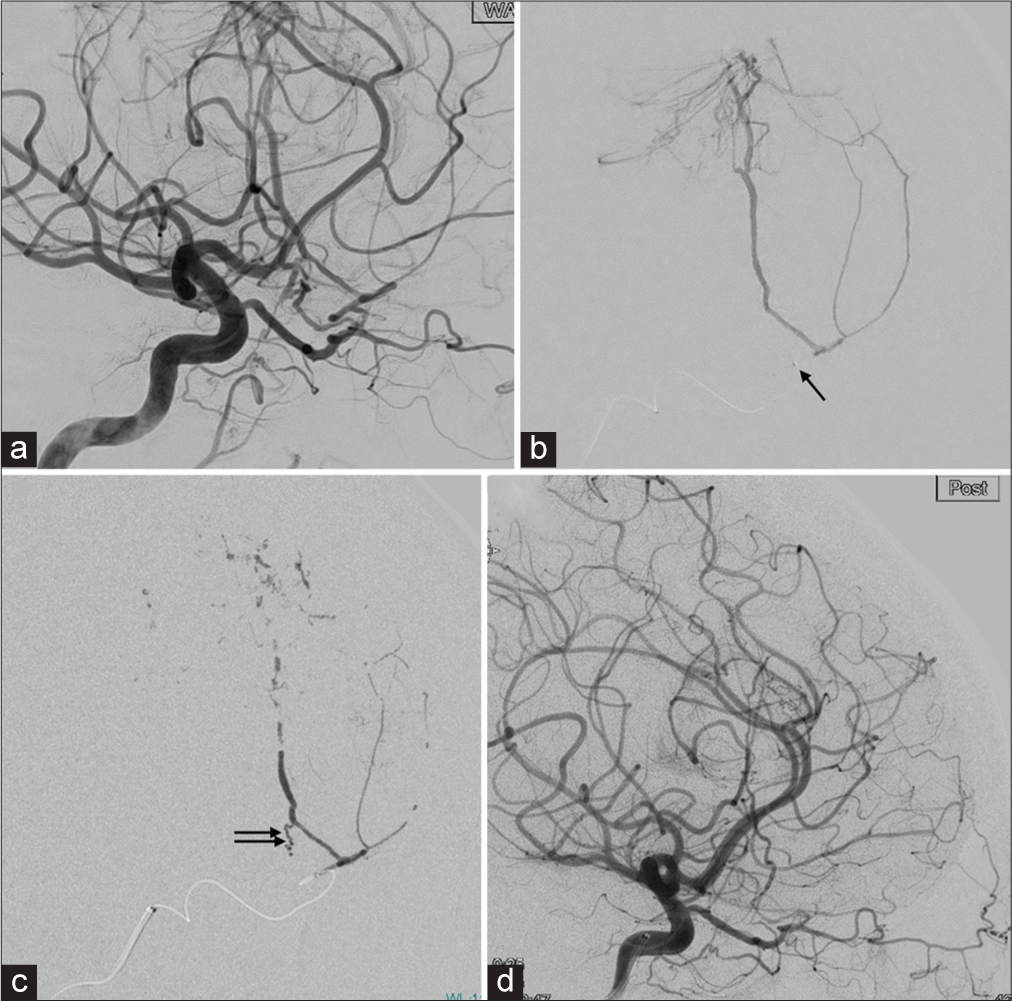
Under general anesthesia, a 6Fr guiding sheath was inserted through the right common femoral artery, and systematic heparinization was performed. Right, ICA angiography and 3D-rotational angiography (3DRA) were performed for diagnosis. A 3.2Fr distal access catheter (DAC)/microcatheter/microguidewire (0.014 inch) was initially used for the approach. As a therapeutic target, the OphA was larger than the ILT for guiding the microcatheter distally. DAC was placed at ICA C3-4, and the microcatheter was easily navigated to the 1st segment of the OphA. Next, a flow-guide microcatheter (DeFrictor 1.3Fr®) and microguidewire 0.010 were used to guide the microcatheter across the 2nd segment to a feeder through the meningolacrimal artery branching from the 3rd segment and a confirmation shot was taken from the microcatheter to confirm that sufficient distance from the 2nd segment had been secured. A total embolic volume of 0.39 ml of 16.7% heated n-butyl cyanoacrylate (NBCA) was injected. The injection was stopped when the NBCA was partially injected retrogradely to another feeder in the process of the NBCA injection. The post-internal carotid angiography (ICAG) and 3D-RA images showed that only the feeder from the ILT remained [
Figure 3:
(a) Right ICA angiography. Working angle. (b) The microcatheter is advanced into the second portion of the OphA (arrow). The microcatheter injection showed only tumor feeders and not reflux to the central retinal artery or feeders from the inferolateral trunk. (c) After the NBCA injection. The cast is shown, and a small amount of NBCA is advanced retrogradely from the inferolateral trunk to the feeder. (d) Post procedural right ICAG. No missing arteries. The OphA, central retinal artery, and inferolateral trunk are clearly visible. ICA: Internal carotid artery, OphA: Ophthalmic artery, NBCA: n-butyl cyanoacrylate, ICAG: Internal carotid angiography.
DISCUSSION
In the preoperative embolization of convexity meningiomas, embolization from the MMA convexity branch is often performed. However, in this case, the main trunk of the MMA was coagulation-cut at the level of the foramen spinosum because a combined petrosal approach was applied 22 years earlier at the time of craniotomy. Therefore, the tumor was supplied with blood flow from the meningolacrimal artery, which is a branch of the OphA, and the anteromedial branch of the ILT, which is a branch of the ICA, through the MMA anastomoses on the dura mater, resulting in a characteristic dual feeding pattern.
Embryology
The hyoid artery from the second aortic arch separates from the ICA to form the stapedial artery (SA). SA is divided into the intracranial supraorbital and extracranial maxillomandibular branches, which exit the cranium through the foramen spinosum. The supraorbital division is distributed within the orbit together with the first branch of the trigeminal nerve, and the maxillomandibular division accompanies the second and third branches of the trigeminal nerve. The supraorbital division runs lateral to the trigeminal ganglion and produces a dural branch of the MMA; the orbital branch of the supraorbital division anastomoses with the OphA to form an arterial ring around the optic nerve and branches distally to the lateral and medial sides to form the frontal and nasociliary branches, respectively. The anastomotic part becomes the second portion of the OphA.
ILT is an important branch of the ICA that serves to anastomose ICA with ECA on the dura mater of the middle cranial fossa from the lateral aspect of the cavernous sinus. ILT is a branch of the C4 segment of ICA and provides blood supply to the 3rd, 4th, and 6th cranial nerves as well as to the Gasserian ganglion. It branches into the anteromedial, anterolateral, posterior, and superior branches, which are anastomosed with the branches of the OphA, internal maxillary artery (IMA), and MMA, respectively.[
MMA-OphA anastomoses
The lacrimal branch of the OphA is connected to the MMA through the cranio-orbital foramen (lacrimal foramen) located on the great wing of the sphenoid bone, 4–12 mm lateral to the SOF. The cranio-orbital foramen is also called the lacrimal foramen, meningolacrimal foramen, Hytrl’s canal, foramen, meningo-orbitale, meningo-orbital foramen, or sphenofrontal foramen. Embryologically, the supraorbital branch of the SA is the orbital branch of the MMA and enters the orbit through the cranio-orbital foramen (or SOF). In one study, the anterior branch (orbital branch) of the MMA passed through the cranio-orbital foramen in 43% of cases, through the SOF in 16%, or through both in 5%.[
In this case, the blood supply demand to the tumor likely increased with the development and growth of the convexity meningioma, leading to the formation of a feeder from the original pre-existing meningolacrimal artery and the anteromedial branch of the ILT through the MMA network on the dura mater [
Figure 4:
Illustrations of the newly developed tumor feeder in this case. (a) Normal anatomy around the orbit and middle fossa. (b) In the previous surgery, MMA at the foramen spinosum was a coagulate cut (cross). The blood flow through the meningolacrimal artery to the MMA was supposed to remain. (c) As the convexity meningioma developed and grew, the vascular demand increased. Therefore, the tumor feeder through both the meningolacrimal artery from the OphA and the dural branch from the anteromedial branch of the ILT. (b and c): Red line (ICA), yellow line (trigeminal nerve), and purple line (blood flows to the tumor from the ICA). ICA: Internal carotid artery, OC: Optic canal, OphA: Ophthalmic artery, SOF: Superior orbital fissure, MLF: Meningolacrimal foramen, FR: Foramen rotundum, FO: Foramen ovale, FS: Foramen spinosum, MMA: Middle meningeal artery, ILT: Inferolateral trunk.
Transarterial embolization through meningolacrimal artery
Intra-navigation through the OphA carries some risk. Successful embolization through the OphA without visual complications has been reported, and the evidence for this practice is insufficient to allow routine use, considering the high risk of visual complications. The central retinal artery branches off from the OphA, and super selective microcatheterization and embolization through the OphA present risks such as arterial dissection or inadvertent reflux into the central retinal artery, elevating the risk of blindness after embolization. The safe point for embolization of the OphA was beyond the second portion. We need to navigate the microcatheter distal to this point. Reflux of the embolic agent should not be allowed close to the origin of the central retinal artery. Distal catheterization of the OphA with a flow-guided microcatheter allows antegrade injection of diluted NBCA with very limited reflux. Guiding the microcatheter into the OphA can sometimes be difficult. Therefore, the use of a DAC is essential, as shown in this case. In some cases, it may be possible to guide the microcatheter into the OphA using the flip-turn technique by balloon inflation near the origin of the OphA. The utilization of balloon microcatheters in this procedure can play a significant role in facilitating super selective catheterization to the OphA and mitigating the risk of embolic agents reflux into the OphA and its branches. The safe point for embolization of the OphA was beyond the second portion. The lacrimal artery represents a security point beyond which no branches of the visual tract can arise. Damage to the visual pathways can be avoided if the catheter is placed distally to this point and the embolic agent is injected without reflux.[
CONCLUSION
Transarterial embolization was successful in the case of a frontal convexity meningioma, which was supplied from both the meningolacrimal artery and the ILT from the ICA. Meticulous and thorough preoperative analysis of angioarchitecture helps neurosurgeons and neuro interventionalists develop treatment strategies. An embryological perspective provides an important clue to understanding the development of angioarchitecture and lowers treatment risks.
Ethical approval
The research/study approved by the Institutional Review Board at Ethics Committee of Tokyo Metropolitan Tama Medical Center, number 3-3, dated April 13, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Diamond MK. Homologies of the meningo-orbital arteries of humans: A reappraisal. J Anat. 1991. 178: 223-41
2. Erturk M, Kayalioglu G, Govsa F, Varol T, Ozgur T. The cranio-orbital foramen, the groove on the lateral wall of the human orbit, and the orbital branch of the middle meningeal artery. Clin Anat. 2005. 18: 10-4
3. Kiyosue H, Tanoue S, Hongo N, Sagara Y, Mori H. Artery of the superior orbital fissure: An undescribed branch from the pterygopalatine segment of the maxillary artery to the orbital apex connecting with the anteromedial branch of the inferolateral trunk. AJNR Am J Neuroradiol. 2015. 36: 1741-7
4. Lasjaunias P, More J, Mink J. The anatomy of the inferolateral trunk (ILT) of the internal carotid artery. Neuroradiology. 1977. 13: 215-20
5. Matsumaru Y, Alvarez H, Rodesch G, Lasjaunias P. Embolisation of branches of the ophthalmic artery. Interv Neuroradiol. 1997. 3: 239-45
6. Perrini P, Cardia A, Fraser K, Lanzino G. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg. 2007. 106: 142-50