- Department of Interventional Radiology, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz, Spain,
- Neurointervention Section, Hospital El Cruce Néstor Kirchner, Florencio Varela,
- Department of Neurosurgery, University of Buenos Aires, Buenos Aires, Argentina.
Correspondence Address:
Luis Alberto Domitrovic Zalocar
Neurointervention Section, Hospital El Cruce Néstor Kirchner, Florencio Varela,
Department of Neurosurgery, University of Buenos Aires, Buenos Aires, Argentina.
DOI:10.25259/SNI_366_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis Alberto Domitrovic Zalocar1, Gustavo Doroszuk2, Javier Goland2,3. Transradial approach and its variations for neurointerventional procedures: Literature review. 15-Aug-2020;11:248
How to cite this URL: Luis Alberto Domitrovic Zalocar1, Gustavo Doroszuk2, Javier Goland2,3. Transradial approach and its variations for neurointerventional procedures: Literature review. 15-Aug-2020;11:248. Available from: https://surgicalneurologyint.com/surgicalint-articles/10209/
Abstract
Background: The transfemoral approach (TFA) has been the standard in neuroradiology over the years. However, the transradial approach (TRA) and its variants offer several benefits over the TFA.
Methods: Review of the literature about TRA and its variations. We present our results for different neurointerventional procedures at our institution between January 2018 and December 2019.
Results: We wrote an educational review describing anatomical and technical aspects, advantages, and complications of this approach. In the past year we increased the percentage of neurointerventional procedures performed through radial or ulnar arteries.
Conclusion: There are clearly proven benefits of employing a wrist approach in patients for neurointerventional procedures and its utilization should especially be considered on a daily basis.
Keywords: Neurointervention, Review, Transradial approach, Ulnar approach, Distal radial approach
INTRODUCTION
The transfemoral approach (TFA) has been the standard in neuroradiology over the years for both diagnostic and therapeutic procedures. However, the transradial approach (TRA) and some variants of it, which are most commonly used in the field of interventional cardiology, offer several benefits over the TFA. Catheterization through the wrist is associated with a lower incidence of major access site-related complications, reduced major bleeding, decreased length of stay, reduced hospital costs, and enhanced patient satisfaction.[
Obstacles toward the transition from TFA to TRA have multiple potential causes. The main one could be relative inexperience with the newer approach, essentially because TFA dominates most interventional neuroradiology training, leading to inexperience, and apprehension with complications and their treatment. Another cause could be the erroneous fear of difficulty navigating to the cerebral vasculature from the wrist. In addition to this, no transradial neurointerventional catheter system is currently available in the market; finally, femoral artery size allows for a wider-diameter catheter for navigation, which could be an advantage during treatment.[
The radial approach to coronary angiography and interventions has been used since it was first described by Campeau in 1989.[
The aim of this paper is to provide a brief anatomical review, describe key technical aspects of the TRA technique and its variants, and discuss its advantages, disadvantages, and possible complications. Our experience with and preferences for the various approaches will be discussed.
UPPER LIMB ARTERY ANATOMY
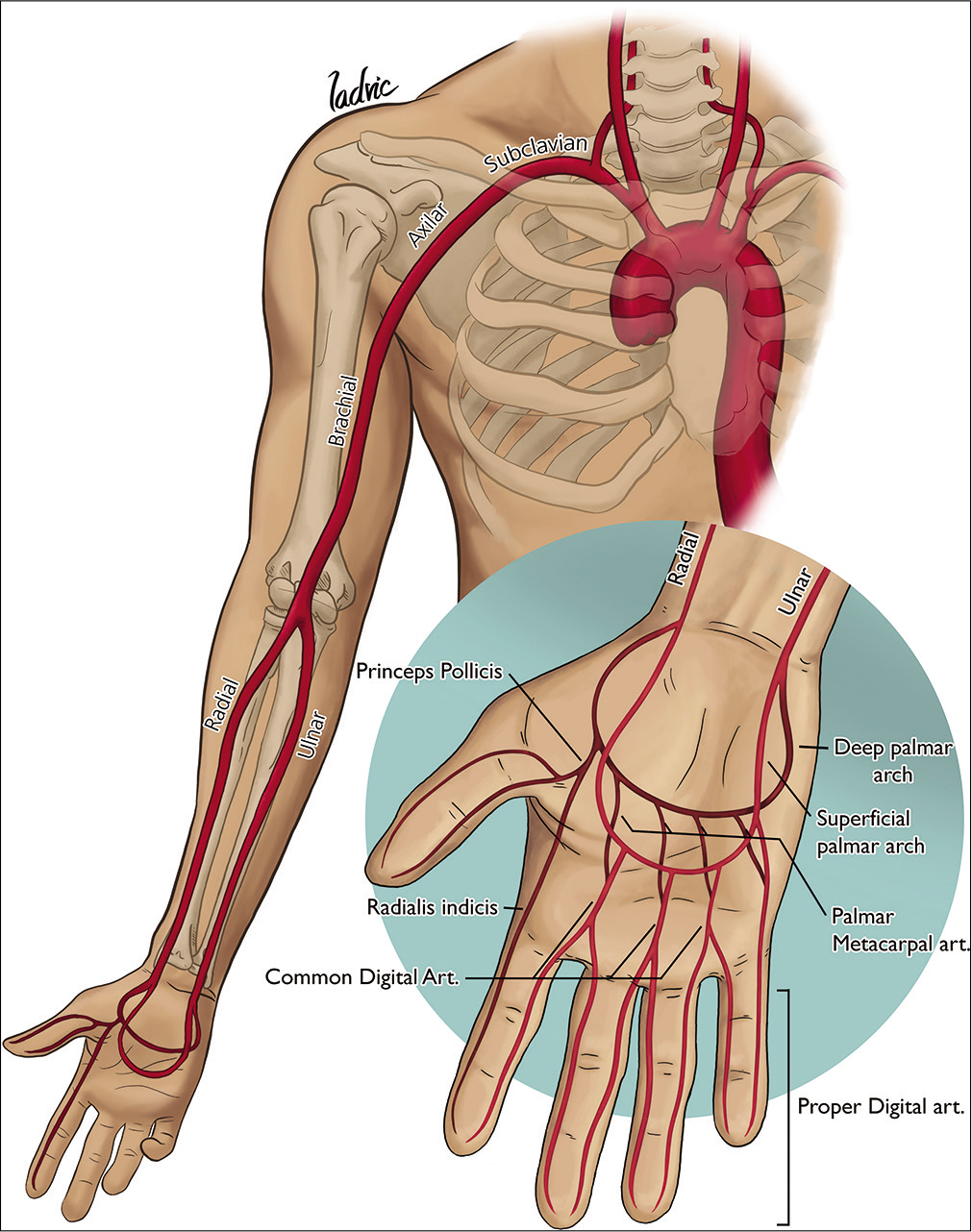
The right subclavian artery is one of the terminal branches of the brachiocephalic artery, whereas on the left side it arises directly as the third branch of the aortic arch. At the level of the first rib, subclavian arteries continue as axillary arteries. The brachial artery is the continuation of the axillary artery after it goes through the inferior edge of the teres major muscle.
The radial artery arises from bifurcation of the brachial artery at the level of the elbow, along with the ulnar artery. The two vessels travel down the radial and ulnar side of the forearm, respectively, to the wrist, where they pass forward into the hand and anastomose with each other through their branches.
Arterial supply to the hand is provided by the superficial and deep palmar arches, which originate from both the ulnar and radial arteries. The superficial arch is typically formed by direct continuity of the ulnar artery with the superficial branch of the radial artery. Four common palmar digital arteries arise from this, each common palmar digital artery giving two proper palmar digital arteries. The deep palmar arch is usually formed by anastomosis between the deep palmar branch of the ulnar artery and the dorsal radial artery. Four palmar metacarpal arteries arise from it, giving contributions to each common digital artery that stems from the superficial arch. The princeps pollicis artery and radialis indicis artery arise from this deep arch [
The patency of the arterial supply of the hand, when radial artery flow is obstructed, depends on the anastomosis between these two arterial arches, specifically whether it is a complete or incomplete arch.[
Radial artery diameter at the wrist in hands with a complete palmar arch is reported to be 3.1 ± 0.2 mm; whereas, for an incomplete arch, the mean diameter is 2.6 ± 0.3 mm.[
Kotowycz et al. found that male sex, wrist circumference, and non-South Asian ancestry were independent predictors of increased radial artery diameter, creating a score (Good Radial Artery Size Prediction – GRASP) that could be used to estimate the size of a patient’s radial artery.[
According to Yan et al., the right ulnar artery is 2.36 ± 0.49 mm and the left ulnar artery is 2.33 ± 0.48 mm; and, as with the radial artery, women tend to have slightly smaller ulnar arteries than men.[
TECHNIQUE
Patient selection
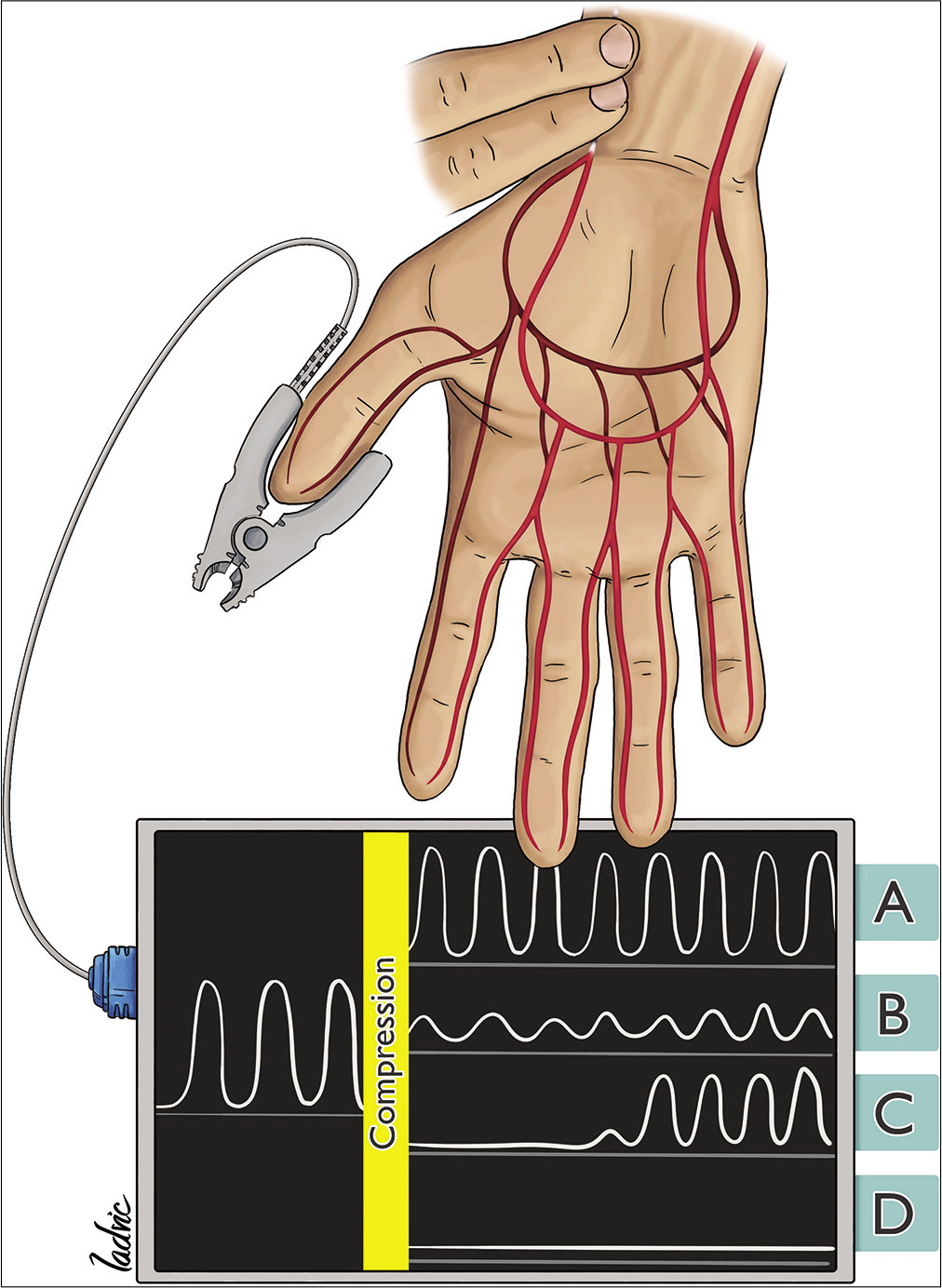
Several tests evaluate for adequate collateral perfusion of the hand. The original Allen’s test was first described in 1929 in three patients with thromboangiitis obliterans by Dr. Allen. It entails compressing the radial artery for 1 min, followed by extending the fingers and watching for the return of color to the hand.[
The original technique was then modified by Wright in the early 1950s and became called the Modified Allen’s test.[
A common practice is to use the modified Allen’s test with a pulse oximeter and plethysmography, also known as the Barbeau test, because it is simple to do, adds little additional cost, makes interpretation more objective, is less dependent on the patient’s cooperation, and is more sensitive than the modified Allen’s test.[
No damping of the pulse tracing immediately after compression Damping of the pulse tracing Loss of the pulse tracing, followed by recovery within 120 s Loss of the pulse tracing, without recovery within 120 s.
Some drawbacks of this test are that the amplitude of the waveform may vary, according to which finger the probe is placed on, proximity of the probe to the arterial source, and the room’s temperature, all of which may influence the characteristics of the waveform.[
However, there is lack of incontrovertible evidence that these tests can predict or reduce symptomatic hand ischemia. An abnormal modified Allen’s test does not necessarily imply that hand ischemia will result if the radial artery is injured.[
Maniotis et al. collected prospective data on 1035 consecutive patients who had undergone TRA procedures performed (94% were performed through the right artery with a 6 Fr sheath) irrespective of the results of Allen’s test, and no significant differences in clinical evolution with or without radial thrombosis were observed.[
Even though most published papers and reviews exclude patients with a negative Allen’s test or a Barbeau type D response,[
We only perform the Barbeau test in patients without a palpable radial pulse in whom we are employing an ulnar artery approach.
Room setup
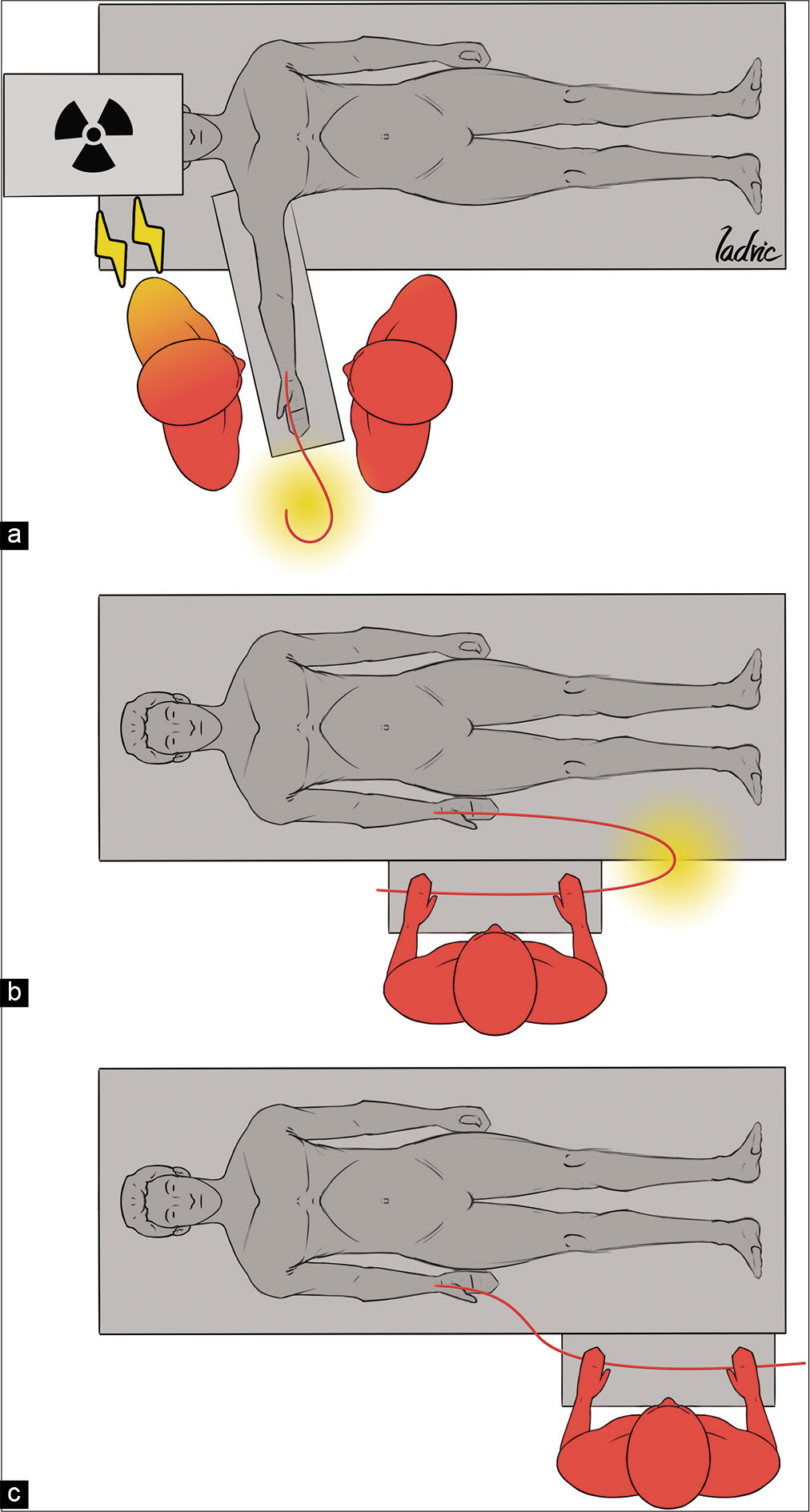
The right radial artery is usually used, due to operator comfort, easier navigation of guiding catheters into the common and internal carotid arteries, and because it allows the clinician to maintain the standard setup for TFA.
The patient’s arm can be positioned in several ways. One option is to position the arm abducted to a 70–90-degree angle. This allows for easier access to the vessel; but it makes catheter exchanges uncomfortable and permits catheters to slide down. Moreover, with this approach, the operator is closer to the X-ray tube.[
Another option is to position the arm in a slightly supine position at the patient’s side, in a position near the patient’s groin, and then elevate the wrist by placing a towel roll underneath. This allows for catheters or wires to be positioned over the patient’s draped body, similar to TFA. However, working with this approach can be cumbersome, as the distal part of the catheter is far away from the operator and must be bent for use.[
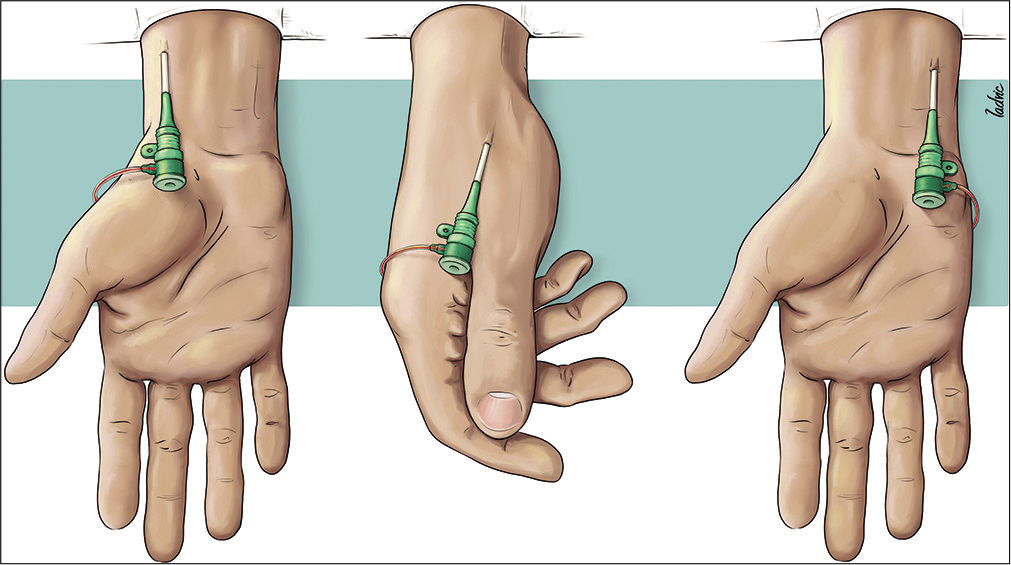
A third possibility is to place a working table distal to the wrist, with the operator standing behind it. We favor this last position, because it allows the operator to stand further from the X-ray tube and helps to keep the catheters and guides straight [
It has been claimed that applying a topical anesthetic and vasodilator cream to the wrist area 30 min before catheterization may facilitate access to the radial artery. Beyer et al, in the PRE-DILATE study, demonstrated that topical application of nitro-glycerine and lidocaine significantly increased radial artery cross-sectional area, with lidocaine also serving as a local anesthetic.[
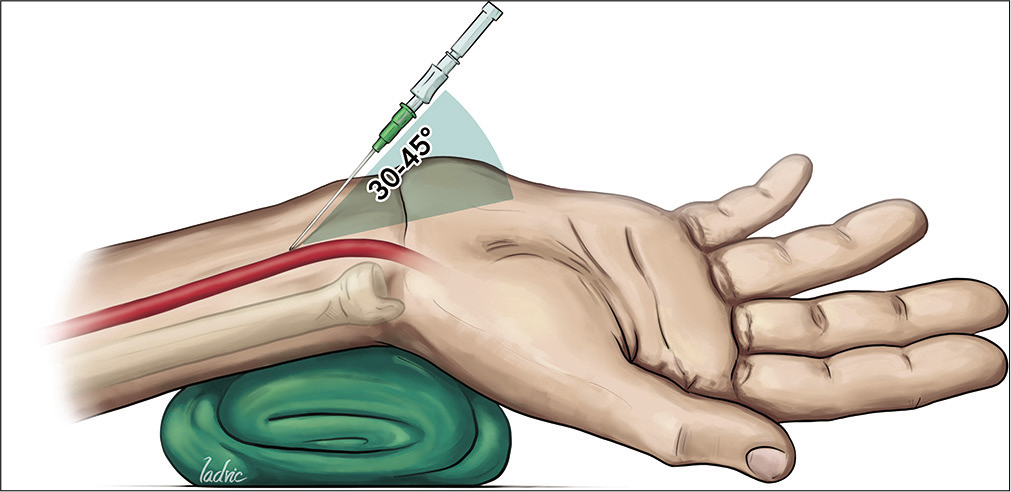
The wrist should be slightly hyperextended, with a towel roll or folded sheet beneath it to support the extended wrist, which brings the artery to the surface and provides gentle tension in the overlying skin, facilitating its puncture.[
There are reports of diagnostic and therapeutic procedures performed through a variation of the TRA, accessing a more distal radial segment at the anatomical snuffbox; this is variably known as the dTRA, snuffbox approach, or very dTRA.[
Another forearm approach is the transulnar artery approach (TUA), which is our third choice in the forearm, after TRA and dTRA. This arterial access technique is similar to the radial approach. TUA, when performed by an experienced operator, has been shown to be non-inferior to the TRA in the cardiac field, with no statistically significant differences in arterial access time, fluoroscopy time, or contrast load, and compared to TRA. This access increases the likelihood of successful forearm access and reduces the need for crossover to the TFA[
Once the vessels are catheterized, the technique is performed similarly to the radial approach.
The skin and periarterial subcutaneous tissues at the puncture site are anesthetized with a subcutaneous anesthetic agent (Xylocaine 1–2%, 0.5–1.5 ml). A 21G micro-puncture kit is used, although 20 G and 18 G needles can also be employed, followed by insertion of the microwire (0.021″) and then a micro-dilator, using the Seldinger technique. A small skin incision at the base of the wire might help with dilatator and sheath insertion. The dilatator is then removed, and a hydrophilic sheath placed, advancing its total length into the artery. Although single-wall entry is ideal, this is usually difficult to achieve, and double-wall puncture is often performed.[
Dedicated radial artery access entry sets, including a 21-gauge needle, 0.018″ micro-guidewire, and sheath (either 4, 5, or 6F) with a dilator tapered to 0.018″, are available.[
Costa et al. claim that an increasing number of puncture attempts required to achieve radial cannulation increases the incidence of radial artery pulsation loss, radial artery obstruction (RAO), pain, and discomfort after each further attempt;[
Rathore et al. have shown that utilizing hydrophilic sheaths decreases the incidence of radial artery spasm (RAS) and pain during TRA, with no difference observed between long and short sheets.[
After sheath placement, and before guide catheter introduction, anti-spasm prophylaxis is performed by instillation. The radial cocktail typically includes nitrates, calcium channel blockers, and heparin to prevent arterial spasm and reduce vascular tone. There is high variability in practice among operators and, despite several recommendations; there is no consensus on the ideal combination. Kwok et al. reported that verapamil, at a dose of 5 mg or verapamil (1.25–5 mg), in combination with nitroglycerine (100-200 μg) is the best combination to reduce RAS.[
Along with the antispasmodic prophylaxis, every patient should receive 5000 UI (70 UI/kg) of heparin after the puncture, but before the beginning of catheter navigation.[
It is important to consider that, if the angiography table does not allow enough lateral movement to include the arm, the system should be advanced gently, without fluoroscopy, to the level of the shoulder, into the subclavian artery, taking care to avoid aberrant anastomotic connections between major arteries in the forearm and brachium.
Catheters and navigation
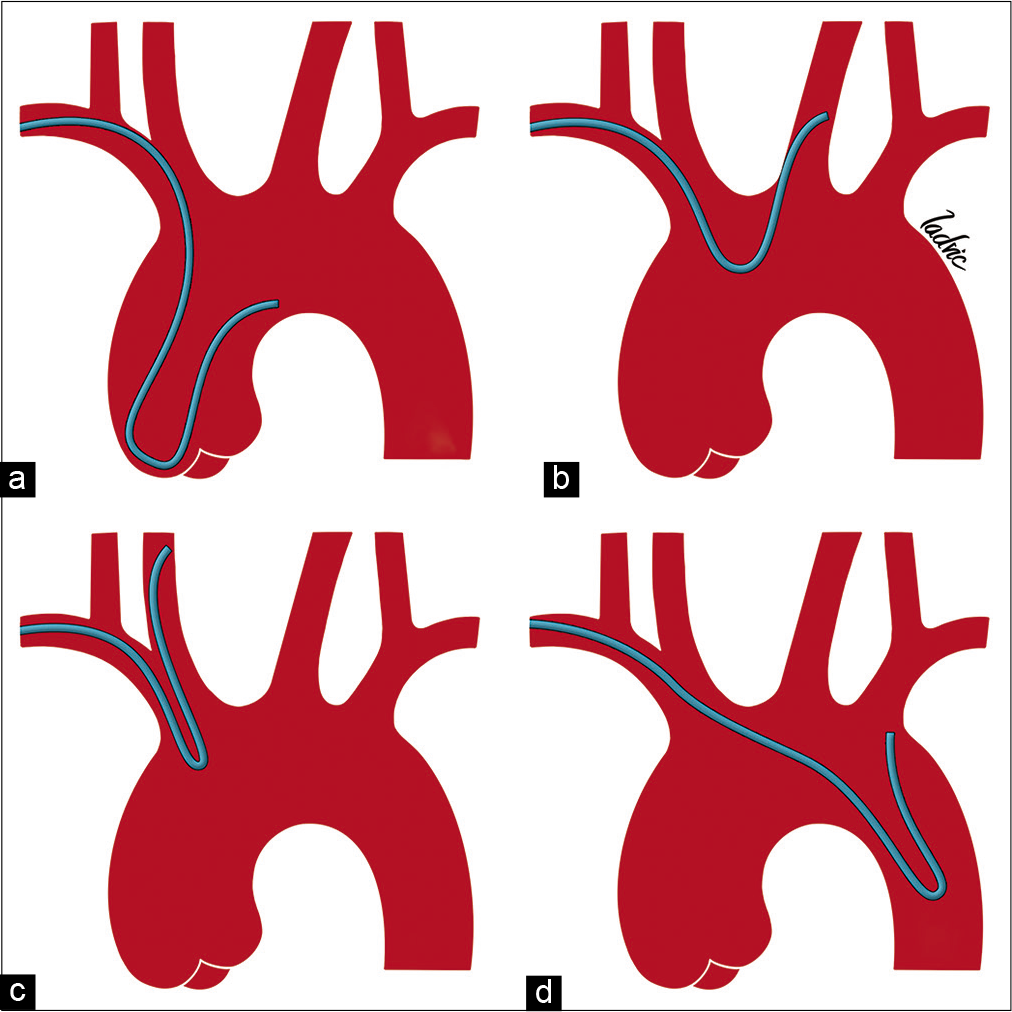
We begin navigation through the vessels, for diagnosis or therapeutic purposes, with a Simmons 2 catheter and 175 cm J-shaped 0.035” wire. The catheter curve may be formed at the aortic valve, ascending aorta, aortic arch, descending aorta or, sometimes, navigating the left carotid artery or right vertebral artery directly with a hydrophilic guide without reforming the catheter.[
Left carotid artery catheterization is easier to accomplish with the formed Simmons catheter placed in the ascending aorta, since the angle formed when the catheter is in place in the descending aorta may not be enough to select this vessel. It is possible to achieve four-vessel catheterization with a Simmons 1, but it becomes more difficult to select the left vertebral and both external carotid arteries.
Once the Simmons catheter is reconstituted, selective four-vessel catheterization is performed identically as in TFA. If selective angiography of the internal or external carotid arteries is necessary, the vessel is navigated using a hydrophilic guide. When angiography is followed by endovascular treatment, the Simmons catheter must be exchanged for another type of catheter. This switch can be done in four different ways:
Using a 260 mm guide inside the external carotid artery for anterior circulation pathology, Using a 260 mm guide inside the left vertebral artery for left posterior inferior cerebellar artery pathology (aneurysms or AVM) or a hypoplastic right vertebral artery, Straight into the right vertebral artery to access other posterior circuit pathology, Through scheduled treatment interventions, where there is no need to perform four-vessel angiography; in such instances, navigation commences with a Chaperon guide catheter over a inner Simmons catheter, followed by direct catheterization of the selected vessel with the guiding catheter.
At this time, a number of different types of therapeutic catheter are available:
Guide catheters: Vertebral or JR catheters, or even a Chaperon guide catheter with the Simmons, with no need to switch to a 260 cm wire. Distal access catheters: This type of catheter is useful for distal pathology or in tortuous or kinked arteries, and preferably used with a proximal carotid sheath. We have performed procedures using Sophia, Fargo max, Catalyst, and Navien catheters without complications. 6-Fr guiding sheaths: This device requires removal of the diagnosis catheter and introducer sheath, but the internal diameter of this guiding sheath permits use of a distal access catheter inside it, with better stability. We only have limited experience with this device for radial access: we used a Shuttle Select Cook sheath for two cases.
Closure
If there is resistance when removing the sheath at the end of the procedure, then a second infusion of antispasmodic should be given.[
Non-occlusive or patent hemostasis is recommended. This consists of compression, and maintaining anterograde flow in the artery, evaluated by plethysmography and is typically described using a wrist band device. There are several wrist band models in the market today. The wrist band is kept in place for from 2 to 3 h, depending on the amount of heparin administered during the procedure and the procedure’s complexity. If continued oozing or bleeding is noted at the time of initial brace removal, an additional 20 min–1 h of bracing is used. Once the band is successfully removed, the patient is observed for 30 min before discharge.[
We use a gauze and an elastic bandage in a hard-compressive way for 1 h, and then leave a soft-compressive bandage in place for 1 day.
ADVANTAGES AND DISADVANTAGES
Advantages
TRA is associated with a lower incidence of major site-related complications, relative to TFA.[
Anatomically, the radial artery is superficial and easily compressed to control bleeding and achieve hemostasis, thereby reducing its potential impact on morbidity and mortality.[
The ulnar artery has been reported to have less anatomical variations with fewer loops and tortuosity than the radial artery. It has also been shown to have fewer adrenergic receptors, thereby reducing the rates of arterial spasm compared to the radial approach, as mentioned in some articles.[
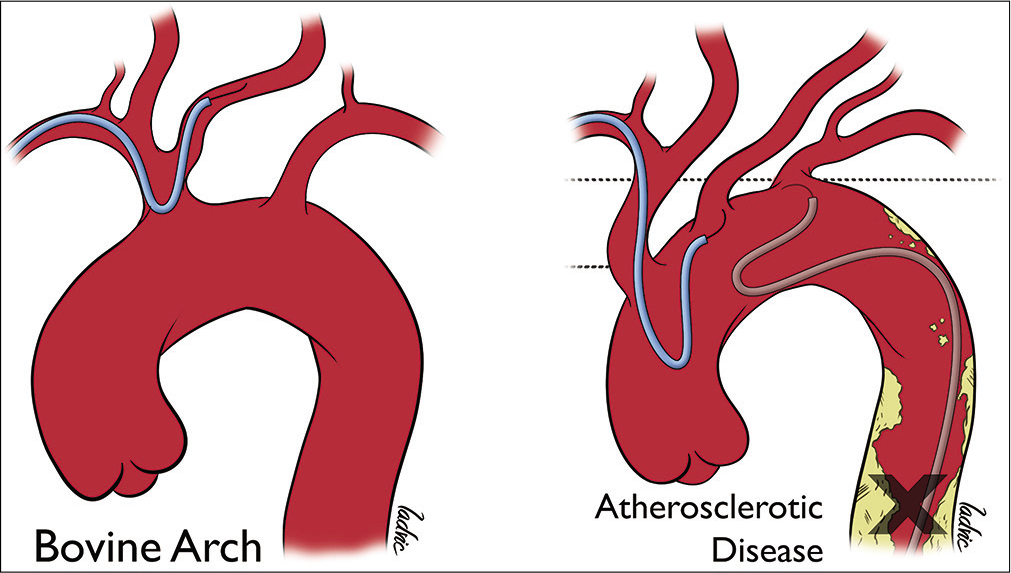
A bovine configuration arch, where the innominate artery and left common carotid artery share a common origin, allows direct catheterization of the latter without needing to enter the aorta or needing to reformat the Simmons catheter curve.[
The TRA may improve guide catheter stability, since the catheters are constrained in relatively small-diameter vessels, compared with TFA, with none of the catheter segment floating in the arch, and leading to less frequent catheter herniation.[
With TFA, a higher rate of complications has been described in patients on anticoagulant and/or antiplatelet therapy. With TRA, there is no need to stop antiplatelet or anticoagulation treatment.[
Subgroups of patients that appear to experience more benefit from TRA over TFA, include obese patients, patients taking anticoagulants, and elderly patients.[
In obese patients, difficulty gaining femoral artery access and achieving hemostasis, and a delay in the recognition of poor hemostasis, leads to an increased risk of vascular complications. A higher risk of complications has been reported among obese patients. The TROP registry showed that, in patients with a BMI >35 kg/m2, employing TRA, versus TFA, reduced the rates of vascular complications delaying hospital discharge and/or transfusion (0.8% vs. 5.1%, P < 0.0009) and hematomas (1.8% vs. 10.2%, P < 0.0001).[
Elderly patients, above 75 years, also have been found to have a lower rate of major complications, like bleeding (that requires surgery or transfusion) or stroke after TRA, compared to TFA (n = 152, 0% vs. 3.2%, P < 0.001).[
In several different surveys, patients preferred TRA over TFA, in terms of quality of life after catheterizations performed for percutaneous coronary interventions and cerebrovascular procedures.[
Satti et al. interviewed patients undergoing diagnostic or interventional cerebrovascular procedures, and found that, out of 25 patients who had undergone both TRA and TFA previously, 24 (96%) preferred having TRA for their next procedure.[
Some authors have suggested that there are economic advantages to the TRA, as demonstrated by a 10–15% cost reduction when compared with TFA for percutaneous cardiac interventions, despite longer procedure times with TRA.[
It also has been reported that TUA, performed by an experienced operator, is non-inferior to TRA for coronary angiographies.[
Disadvantages
The TRA and it variations require a new learning curve, due to specific techniques and anatomical difficulties that are usually overcome with experience, resulting in longer procedural times, greater radiation exposure, and a higher rate of crossover to another approach.[
Some investigators have found that procedural failure and site crossover were higher in those for whom TRA versus TFA was used,[
Large randomized multicenterd trials have been conducted at centers at which a high proportion of procedures employ a radical approach and have demonstrated a benefit of radial femoral access, in terms of access-site crossover, major vascular complications, and the composite of death, myocardial infarction and stroke. Meanwhile, femoral access was not found to be superior to radial access at high-volume femoral access centers. The effectiveness of TRA might, therefore, be linked to expertise and volume.[
Anatomical variations in the arm or subclavian tortuosity may present challenges, especially during the learning curve. The anatomical variant called the lusoria artery, whereby the right subclavian artery arises as a fourth branch of the aorta, greatly increases the technical challenge of performing TRA, and might even require crossover to complete the cerebral angiogram.[
Large catheters cannot be used in patients with small radial arteries, especially women, elderly patients, and very low- weight patients. Their use may generate pain due to major friction and increase the risks of radial spasm, arterial trauma, and potential arterial occlusion.[
In transradial cerebral angiography, if selection of the left ICA, left subclavian artery or left VA is required, a right TRA procedure may be more difficult in some patients.[
The TFA should, therefore, be the approach of choice over TRA in:
Patients with cardiogenic shock without a palpable radial pulse[ Patients at risk of needing hemodialysis, since the radial artery must be preserved for a potential arteriovenous fistula[ Patients needing coronary or cerebral revascularization using the radial artery as a donor graft.[
COMPLICATIONS
Although the TRA is associated with a low morbidity rate, potentially-severe complications include: arterial occlusion, arterial dissection, RAS, arteriovenous fistula, and pseudoaneurysm formation; though the majority of complications can be successfully treated with conservative management without permanent disability.[
In one study, there was no statistically significant difference in the incidence of major adverse cardiac events between patients who underwent catheterization through TUA versus TRA. Furthermore, there was no significant difference in the incidence of bleeding or hematoma formation, or of vasospasm or arterial occlusion between these two access routes.[
Hematoma
The most common minor complication with TRA, sometimes not reported, is a localized minor hematoma or ecchymosis associated with the puncture site and associated pain.[
Hematoma of the arm proximal to the elbow that is non-access site related but secondary to small branch perforation or artery laceration must be detected promptly to ensure rapid management. In case of a large or growing hematoma, a blood pressure cuff should be rapidly inflated at the hematoma site to 20 mmHg below systolic pressure for 15 min and repeated, if needed, to facilitate sealing of the perforation.[
Arm hematoma and compartment syndrome are extremely rare, occurring in <0.01% of patients undergoing catheterization through TRA, and generally published as case reports.[
Radial Artery Occlusion (RAO)
Reported RAO rates range from 3% to 30%. It is a clinically- silent complication of TRA in properly selected cases, but more commonly impedes future utilization of the radial artery.[
In the RIVAL trial – a randomized, parallel group, multicenter study – symptomatic radial occlusion requiring medical attention and ultrasound confirmation occurred after just 0.2% of procedures (n = 6/3507) and no patients required surgical intervention.[
Uhlemann et al. detected a higher rate than expected, with RAO occurring in 13.7% of patients with 5-Fr sheaths and 30.5% with 6-Fr sheaths (P < 0.001, n = 455). Of all the patients with RAO, 42.5% (n = 48) were symptomatic within 24 h, and an additional 7% became symptomatic within a mean of 4 days after catheterization. The most frequent symptoms were painful forearm and thenar eminence. Other symptoms were a loss of handgrip force and paraesthesia. Critical limb ischemia did not occur in any patient.[
In a systematic review and meta-analysis published by Polimeni et al., a significantly-reduced incidence of bleeding events was identified when 5Fr versus 6Fr catheters were used, but there was no significant difference in RAO incidence between these catheters. However, upon meta-regression, an increasing benefit was evident with 5Fr sheaths as the percentage of women included into the study increased, which could be a consequence of women’s reduced radial artery diameter.[
A higher number of puncture attempts required to achieve radial cannulation is linked to an increased risk of RAO,[
Digital ischemia is exceedingly rare, described in the literature in critical patients without an ulnopalmar arch.[
Radial Artery Spasm (RAS)
RAS may cause patient discomfort and lead to procedure failure. Angiographic confirmation is important, since pain in the arm might sometimes not be caused by spasm, but by other factors such as tortuosity/loops in the radial, brachial, or subclavian arteries, which renders catheter movement difficult and causes the patient pain.
Jo et al. documented RAS in 174 of 1240 procedures (14%).[
If intraprocedural RAS occurs, in most cases, it can be effectively resolved through the administration of medication to relieve anxiety and a calcium-channel blocker and/or nitroglycerine.[
Two methods have been described that could prevent the occurrence of spasm: one non-pharmacological, by providing the patient with clear and reassuring information to reduce the stress-related risk of radial spasm; and one pharmacological, by administering a vasodilating cocktail and intravenous sedation.[
When difficulties are encountered during wire progression, an angiographic assessment is highly recommended to better understand the problem and guide management. Some sources of resistance advancing the catheter to consider, especially at the level of the elbow, are arterial spasm, vessel tortuosity, and anatomical variations secondary to a remnant artery.[
EL CRUCE HOSPITAL EXPERIENCE
Between January 1 and December 31, 2019, we performed 301 endovascular procedures, of which 187 were through upper extremity access (62.13%, including 160 TRA, 19 dTRA, and 8 TUA). A total of 91 embolization procedures were performed, of which 53 were performed by TRA and five by TUA (63.7% of the total therapeutic procedures were performed through the wrist). Two hundred and ten diagnostic procedures were performed, of which 130 were performed through the wrist (61.9%).
Over the same period of time, but in 2018, the percentage of diagnostic and therapeutic procedures combined performed through the wrist was 51.9% so that the percentage of total procedures performed through wrist access increased by more than 10% over a single year.
We have experienced a very low rate of complications. In two patients, we had to cross over to TFA for technical difficulties: one because of an excessively-elongated arc and a second due to lusoria artery variation. Three patients presented with severe RAS that forced us to perform TFA. Another complication was radial dissection in two patients, who remained asymptomatic; their studies ultimately were performed through TFA. We also experienced an arteriovenous fistula due to TRA, which only was detected because a murmur was detected 1 week after the procedure, without hemodynamic repercussions. Treatment in this case was conservative and the fistula closed spontaneously within 6 months.
In seven patients, we had to cross over from wrist to TFA, six of these in 2018. This translates into a 1.19% failed wrist approach rate over 2 years.
Every year, we increase the number of endovascular approaches that we are performing through the wrist, increasing procedural effectiveness due to a reduced rate of complications and increased patient comfort.
CONCLUSION
There are clearly-proven benefits of employing a wrist approach over TFA in selected patients, and its utilization should especially be considered for neurointerventions on a daily basis. Continuous use of this approach increases operator comfort and, hence, the percentage of successful procedures that can be performed through the wrist.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achenbach S, Ropers D, Kallert L, Turan N, Krähner R, Wolf T. Transradial versus transfemoral approach for coronary angiography and intervention in patients above 75 years of age. Catheter Cardiovasc Interv. 2008. 72: 629-35
2. Allen EV. Thromboangeitis obliterans: Methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am J Med Sci. 1929. 178: 237-44
3. Barbeau GR, Arsenault F, Dugas L, Simard S, Larivière MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: Comparison with the Allen’s test in 1010 patients. Am Heart J. 2004. 147: 489-93
4. Benamer H, Louvard Y, Sanmartin M, Valsecchi O, Hildick-Smith D, Garot P. A multicentre comparison of transradial and transfemoral approaches for coronary angiography and PTCA in obese patients: The TROP registry. Eurointervention. 2007. 3: 327-32
5. Beyer AT, Ng R, Singh A, Zimmet J, Shunk K, Yeghiazarians Y. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: A randomized, placebo-controlled, double-blind clinical trial. Int J Cardiol. 2013. 168: 2575-8
6. Biederman DM, Marinelli B, O’Connor PJ, Titano JJ, Patel RS, Kim E. Transradial access for visceral endovascular interventions in morbidly obese patients: Safety and feasibility. J Vasc Access. 2016. 17: 256-60
7. Bilge O, Pinar Y, Özer MA, Gövsa F. A morphometric study on the superficial palmar arch of the hand. Surg Radiol Anat. 2006. 28: 343-50
8. Brener MI, Bush A, Miller JM, Hasan RK. Influence of radial versus femoral access site on coronary angiography and intervention outcomes: A systematic review and meta-analysis. Catheter Cardiovasc Interv. 2017. 90: 1093-104
9. Brunet MC, Chen SH, Sur S, McCarthy DJ, Snelling B, Yavagal DR. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J Neurointerv Surg. 2019. 11: 710-3
10. Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: A comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009. 109: 1763-81
11. Buch C, Devora CM, Johnson LY, Rahimi OB, Kar R. Incomplete superficial palmar arch and bilateral persistent median artery. Int J Surg Case Rep. 2019. 58: 205-7
12. Cauley R, Wu WW, Doval A, Chaikof E, Ho KKL, Iorio ML. Identifying complications and optimizing consultations following transradial arterial access for cardiac procedures. Ann Vasc Surg. 2019. 56: 87-96
13. Cheaito R, Benamer H, Hovasse T, Tritar A, Hage F, Garot P. Feasibility and safety of transradial coronary interventions using a 6.5-F sheathless guiding catheter in patients with small radial arteries. Catheter Cardiovasc Interv. 2015. 86: 51-8
14. Chen CW, Lin CL, Lin TK, Lin CD. A simple and effective regimen for prevention of radial artery spasm during coronary catheterization. Cardiology. 2005. 105: 43-7
15. Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A. Effect of transradial access on quality of life and cost of cardiac catheterization: A randomized comparison. Am Heart J. 1999. 138: 430-6
16. Costa F, van Leeuwen MA, Daemen J, Diletti R, Kauer F, van Geuns RJ. The rotterdam radial access research: Ultrasound-based radial artery evaluation for diagnostic and therapeutic coronary procedures. Circ Cardiovasc Interv. 2016. 9: e003129
17. DeBucourt M, Teichgräber U. Digital ischemia and consecutive amputation after emergency transradial cardiac catheter examination. Cardiovasc Intervent Radiol. 2012. 35: 1242-4
18. Fernandez R, Zaky F, Ekmejian A, Curtis E, Lee A. Safety and efficacy of ulnar artery approach for percutaneous cardiac catheterization: Systematic review and meta-analysis. Catheter Cardiovasc Interv. 2018. 91: 1273-80
19. Fischman AM, Swinburne NC, Patel RS. A technical guide describing the use of transradial access technique for endovascular interventions. Tech Vasc Interv Radiol. 2015. 18: 58-65
20. Frangos C, Noble S. How to transform you into a radialist (Part II): Tips and tricks. Cardiovasc Med. 2011. 14: 315-24
21. Frangos C, Noble S. How to transform you into a radialist: Literature review. Cardiovasc Med. 2011. 14: 277-82
22. Fuhrman TM, Pippin WD, Talmage LA, Reilley TE. Evaluation of collateral circulation of the hand. J Clin Monit. 1992. 8: 28-32
23. Gellman H, Botte MJ, Shankwiler J, Gelberman RH. Arterial patterns of the deep and superficial palmar arches. Clin Orthop Relat Res. 2001. 383: 41-6
24. Ghuran AV, Dixon G, Holmberg S, de Belder A, Hildick-Smith D. Transradial coronary intervention without pre-screening for a dual palmar blood supply. Int J Cardiol. 2007. 121: 320-2
25. Gokhroo R, Bisht D, Padmanabhan D, Gupta S, Kishor K, Ranwa B. Feasibility of ulnar artery for cardiac catheterization: AJmer ULnar ARtery (AJULAR) catheterization study. Catheter Cardiovasc Interv. 2015. 86: 42-8
26. Goland J, Domitrovic L, Doroszuk G, Garbugino S, Ypa P. Distal radial approach for neurointerventional diagnosis and therapy. Surg Neurol Int. 2019. 10: 1-5
27. Goland J, Doroszuk G. Transradial approach for endovascular diagnosis and treatment of ruptured cerebral aneurysms: A descriptive study. Surg Neurol Int. 2019. 10: 87
28. Goland J, Doroszuk GF, Garbugino SL, Ypa MP. Transradial approach to treating endovascular cerebral aneurysms: Case series and technical note. Surg Neurol Int. 2017. 8: 73
29. Habib J, Baetz L, Satiani B. Assessment of collateral circulation to the hand prior to radial artery harvest. Vasc Med. 2012. 17: 352-61
30. Hahalis G, Deftereos S, Bertrand OF. Ulnar artery: The ulysses ultimate resort for coronary procedures. Hell J Cardiol. 2016. 57: 238-46
31. Haussen DC, Nogueira RG, DeSousa KG, Pafford RN, Janjua N, Ramdas KN. Transradial access in acute ischemic stroke intervention. J Neurointerv Surg. 2016. 8: 247-50
32. Hibbert B, Simard T, Wilson KR, Hawken S, Wells GA, Ramirez FD. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. JACC Cardiovasc Interv. 2012. 5: 819-26
33. Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: Incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med. 2012. 13: 193-5
34. Jo KW, Park SM, Kim SD, Kim SR, Baik MW, Kim YW. Is transradial cerebral angiography feasible and safe? rtdA single center’s experience. J Korean Neurosurg Soc. 2010. 47: 332-7
35. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet. 2011. 377: 1409-20
36. Kiberenge RK, Ueda K, Rosauer B. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: A randomized controlled trial. Anesth Analg. 2018. 126: 120-6
37. Kok MM, Weernink MG, von Birgelen C, Fens A, van der Heijden LC, van Til JA. Patient preference for radial versus femoral vascular access for elective coronary procedures: The prevas study. Catheter Cardiovasc Interv. 2018. 91: 17-24
38. Kotowycz MA, Johnston KW, Ivanov J, Asif N, Almoghairi AM, Choudhury A. Predictors of radial artery size in patients undergoing cardiac catheterization: Insights from the good radial artery size prediction (GRASP) study. Can J Cardiol. 2014. 30: 211-6
39. Kwok CS, Rashid M, Fraser D, Nolan J, Mamas M. Intra-arterial vasodilators to prevent radial artery spasm: A systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. 2015. 16: 484-90
40. Layton KF, Kallmes DF, Cloft HJ. The radial artery access site for interventional neuroradiology procedures. AJNR Am J Neuroradiol. 2006. 27: 1151-4
41. Lee DG, Lee DH, Shim JH, Suh DC. Feasibility of the transradial or the transbrachial approach in various neurointerventional procedures. Neurointervention. 2015. 10: 74-81
42. Levy EI, Boulos AS, Fessler RD, Bendok BR, Ringer AJ, Kim SH. Transradial cerebral angiographý: An alternative route. Neurosurgery. 2002. 51: 335-42
43. Lin YJ, Chu CC, Tsai CW. Acute compartment syndrome after transradial coronary angioplasty. Int J Cardiol. 2004. 97: 311
44. Louvard Y, Lefevre T, Allain A, Morice MC. Coronary angiography through the radial or the femoral approach: The carafe study. Catheter Cardiovasc Interv. 2001. 52: 181-7
45. Maniotis C, Koutouzis M, Andreou C, Lazaris E, Tsiafoutis I, Zografos T. Transradial approach for cardiac catheterization in patients with negative Allen’s test. J Invasive Cardiol. 2015. 27: 416-20
46. Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: A scientific statement from the American heart association. Circ Cardiovasc Interv. 2018. 11: 1-21
47. McDonagh JR, Seth M, Lalonde TA, Khandewal AK, Wohns DH, Dixon SR. Radial PCI and the obesity paradox: Insights from blue cross blue shield of Michigan cardiovascular consortium (BMC2). Catheter Cardiovasc Interv. 2016. 87: 211-9
48. Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic review and cost-benefit analysis of radial artery access for coronary angiography and intervention. Circ Cardiovasc Qual Outcomes. 2012. 5: 454-62
49. Nohara AM, Kallmes DF. Transradial cerebral angiography: Technique and outcomes. AJNR Am J Neuroradiol. 2003. 24: 1247-50
50. Ouadhour A, Sideris G, Smida W, Logeart D, Stratiev V, Henry P. Usefulness of subcutaneous nitrate for radial access. Catheter Cardiovasc Interv. 2008. 72: 343-6
51. Pancholy SB, Patel TM. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter Cardiovasc Interv. 2012. 79: 78-81
52. Polimeni A, Passafaro F, De Rosa S, Sorrentino S, Torella D, Spaccarotella C. Clinical and procedural outcomes of 5-french versus 6-french sheaths in transradial coronary interventions. Medicine (Baltimore). 2015. 94: e2170
53. Ranwa B, Priti K. Transulnar versus transradial access as a default strategy for percutaneous coronary intervention. Heart Views. 2019. 20: 152-7
54. Rathore S, Stables RH, Pauriah M, Hakeem A, Mills JD, Palmer ND. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: A randomized study. JACC Cardiovasc Interv. 2010. 3: 475-83
55. Roghani-Dehkordi F, Mansouri R, Khosravi A, Mahaki B, Akbarzadeh M, Kermani-Alghoraishi M. Transulnar versus transradial approach for coronary angiography and angioplasty: Considering their complications. ARYA Atheroscler. 2018. 14: 128-31
56. Roh JH, Lee JH. Distal radial approach through the anatomical snuff box for coronary angiography and percutaneous coronary intervention. Korean Circ J. 2018. 48: 1131-4
57. Satti SR, Vance AZ, Golwala SN, Eden T. Patient preference for transradial access over transfemoral access for cerebrovascular procedures. J Vasc Interv Neurol. 2017. 9: 1-5
58. Seto AH, Roberts JS, Abu-Fadel MS, Czak SJ, Latif F, Jain SP. Real-time ultrasound guidance facilitates transradial access. JACC Cardiovasc Interv. 2015. 8: 283-91
59. Snelling BM, Sur S, Shah SS, Caplan J, Khandelwal P, Yavagal DR. Transradial approach for complex anterior and posterior circulation interventions: Technical nuances and feasibility of using current devices. Oper Neurosurg (Hagerstown). 2019. 17: 293-302
60. Snelling BM, Sur S, Shah SS, Khandelwal P, Caplan J, Haniff R. Transradial cerebral angiography: Techniques and outcomes. J Neurointerv Surg. 2018. 10: 874-81
61. Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, Van Der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997. 40: 156-8
62. Sur S, Snelling B, Khandelwal P, Caplan JM, Peterson EC, Starke RM. Transradial approach for mechanical thrombectomy in anterior circulation large-vessel occlusion. Neurosurg Focus. 2017. 42: E13
63. Uhlemann M, Möbius-Winkler S, Mende M, Eitel I, Fuernau G, Sandri M. The leipzig prospective vascular ultrasound registry in radial artery catheterization. JACC Cardiovasc Interv. 2012. 5: 36-43
64. Valgimigli M, Gagnor A, Calabró P, Frigoli E, Leonardi S, Zaro T. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: A randomised multicentre trial. Lancet. 2015. 385: 2465-76
65. Wretowski D, Krakowian M, Łabyk A, Pruszczyk P, Roik M. Very distal transradial approach (VITRO) for coronary interventions. Postepy Kardiol Interwencyjnej. 2019. 15: 42-5
66. Yan ZX, Zhou YJ, Zhao YX, Zhou ZM, Yang SW, Wang ZJ. Anatomical study of forearm arteries with ultrasound for percutaneous coronary procedures. Circ J. 2010. 74: 686-92
67. Yoo BS, Yoon J, Ko JY, Kim JY, Lee SH, Hwang SO. Anatomical consideration of the radial artery for transradial coronary procedures: Arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005. 101: 421-7
68. Ziakas AG, Koskinas KC, Gavrilidis S, Giannoglou GD, Hadjimiltiades S, Gourassas I. Radial versus femoral access for orally anticoagulated patients. Catheter Cardiovasc Interv. 2010. 76: 493-9