- Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka, Japan.
- Department of Neurosurgery, The University of Tokyo Hospital, Bunkyo-ku, Japan.
- Department of Neurosurgery, NTT Medical Center Tokyo, Shinagawa-ku, Tokyo, Japan.
Correspondence Address:
Hideaki Ono
Department of Neurosurgery, Fuji Brain Institute and Hospital, Fujinomiya, Shizuoka, Japan.
DOI:10.25259/SNI_462_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Seiei Torazawa, Hideaki Ono, Tomohiro Inoue, Takeo Tanishima, Akira Tamura, Isamu Saito. Trapping, dome puncture, and direct suction decompression in conjunction with assistant superficial temporal artery- middle cerebral artery bypass to clip giant internal carotid artery bifurcation aneurysm. 18-Oct-2019;10:205
How to cite this URL: Seiei Torazawa, Hideaki Ono, Tomohiro Inoue, Takeo Tanishima, Akira Tamura, Isamu Saito. Trapping, dome puncture, and direct suction decompression in conjunction with assistant superficial temporal artery- middle cerebral artery bypass to clip giant internal carotid artery bifurcation aneurysm. 18-Oct-2019;10:205. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9717
Abstract
Background: Very large and giant aneurysms (≥20 mm) of the internal carotid artery (ICA) bifurcation (ICAbif) are definitely rare, and optimal treatment is not established. Endovascular treatments are reported as suboptimal due to difficulties of complete occlusion and tendencies to recanalization. Therefore, direct surgery remains an effective strategy if the clipping can be performed safely and reliably, although very difficult.
Case Description: Two cases of ICAbif aneurysms (>20 mm) were treated. Prior assistant superficial temporal artery (STA)-middle cerebral artery (MCA) bypass was performed to avoid ischemic complications during prolonged temporary occlusion of the arteries in both cases. In Case 1 (22-mm aneurysm), the dome was inadvertently torn in applying the clip because trapping had resulted in insufficient decompression. Therefore, in Case 2 (28-mm aneurysm), almost complete trapping of the aneurysm and subsequent dome puncture was performed, and the aneurysm was totally deflated by suction from the incision. This complete aneurysm decompression allowed safe dissection and successful clipping.
Conclusion: Trapping, deliberate aneurysm dome puncture, and suction decompression from the incision in conjunction with assistant STA-MCA bypass can achieve complete aneurysm deflation, and these techniques enable safe dissection of the aneurysm and direct clipping of the aneurysm neck. Direct clipping with this technique for very large and giant ICAbif aneurysms may be the optimal treatment choice with the acceptable outcome if endovascular treatment remains suboptimal.
Keywords: Dome puncture, Giant aneurysm, Internal carotid artery bifurcation aneurysm, Suction decompression, Trapping
INTRODUCTION
Internal carotid artery (ICA) bifurcation (ICAbif) aneurysms are not common, accounting for about 2–9% of all intracranial aneurysms.[
Despite modern endovascular techniques, coil embolization for ICAbif aneurysms is suboptimal due to the unfavorable configuration of the ICAbif, which causes much hemodynamic stress.[
We recently treated 2 cases of ICAbif aneurysms of more than 20 mm with surgical clipping. In both cases, surgical clipping was successfully achieved finally, but many challenges had to be overcome. We clarify the critical steps and consider the optimal strategy in surgical clipping for very large and giant ICAbif aneurysms.
PREOPERATIVE PRESENTATIONS
Case 1
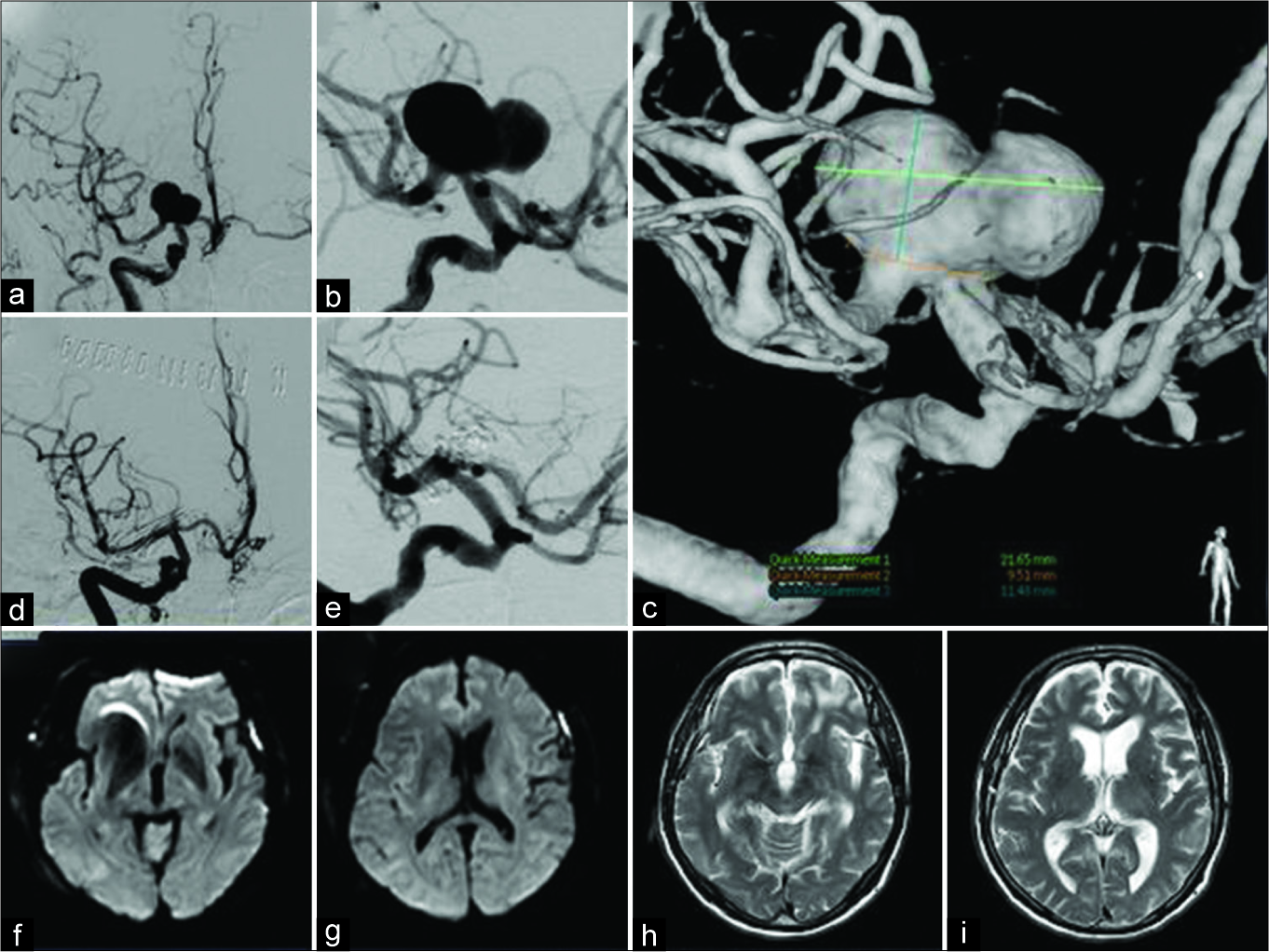
A 69-year-old man presented with dysarthria and transient right hemiparesis. Head magnetic resonance imaging revealed left subcortical infarctions, and magnetic resonance angiography identified a large aneurysm of the right ICA. Digital subtraction angiography (DSA) demonstrated a very large aneurysm of the right ICAbif, with a maximum diameter of 22 mm [
Figure 1:
Case 1 – preoperative digital subtraction angiography (DSA) showing a very large aneurysm of the right internal carotid artery bifurcation of 22 mm maximum diameter (a-c). Postoperative DSA showing complete obliteration of the aneurysm (d and e). Diffusion- weighted imaging on postoperative day 1 showing no infarction (f and g). T2-weighted imaging at 6 months after the operation indicating no new lesion (h and i).
Case 2
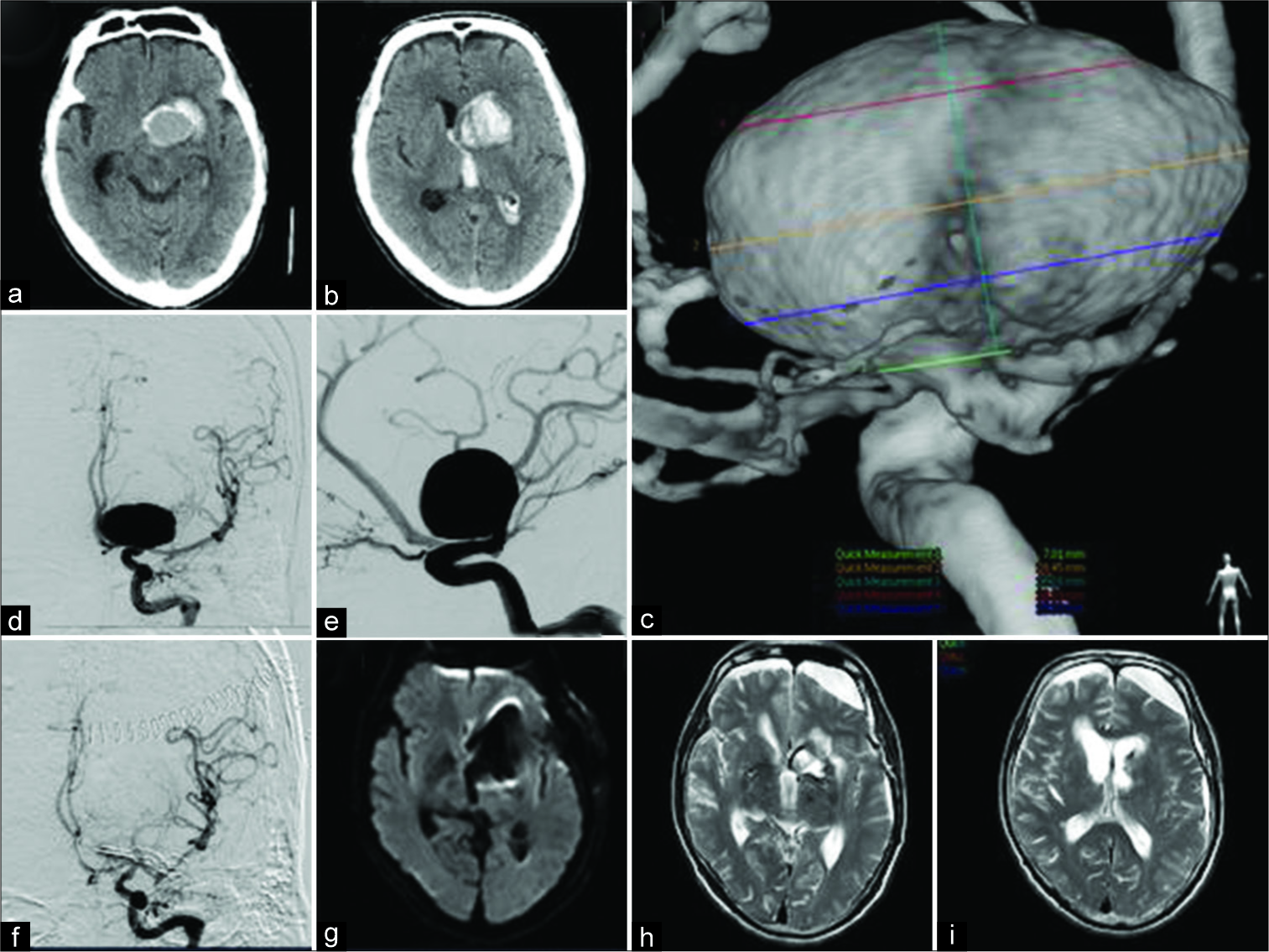
A 67-year-old man presented with sudden right hemiparesis and aphasia. Head computed tomography revealed left frontal intracranial hematoma centered on the caudate nucleus and intra-ventricular hemorrhage, and a suspected large mass lesion in the hematoma [
Figure 2:
Case 2 – head computed tomography on the day of admission revealing left frontal intracranial hematoma centered on the caudate nucleus and intra-ventricular hemorrhage, and a suspected large mass lesion in the hematoma (a and b). Digital subtraction angiography (DSA) showing a 28-mm giant aneurysm of the left internal carotid artery bifurcation (c and e). Postoperative DSA showing complete obliteration of the aneurysm (f). Diffusion-weighted imaging on postoperative day 1 showing no infarction (g). T2-weighted imaging at 6 months after the operation indicating no new lesion (h and i).
SURGICAL STRATEGY
Surgical clipping of very large or giant ICAbif aneurysm requires reduction of blood flow and pressure in the aneurysm by parent artery occlusion to prevent uncontrolled intraoperative rupture of aneurysm and to safely dissect the aneurysm from the surrounding structures. Occlusion time of the parent artery tends to be prolonged in such cases, so we planned to conduct superficial temporal artery (STA)- middle cerebral artery (MCA) bypass before clipping of the aneurysm to maintain adequate blood flow and avoid ischemic complications caused by temporary occlusion of the ICA.
OPERATION
Common steps in both cases
Neuroanesthesia was induced under the monitoring of somatosensory evoked potentials of the contralateral extremities and motor evoked potentials of the upper limb.
First, the cervical ICA was exposed through a short linear skin incision just across the medial edge of the sternocleidomastoid muscle. After the introduction of the operating microscope, the parietal branch of STA was harvested meticulously, and its lumen was filled with heparinized normal saline after cutting the distal end. Standard frontotemporal craniotomy was made, and the sphenoid ridge was drilled to flatten the lateral orbital wall and expose the temporal tip. Adequate drilling of the sphenoid ridge is critical to obtain the anterior-temporal view and clearly visualize the retro-carotid space and ICAbif aneurysm buried in the frontal lobe without severe retraction of the frontal lobe. Before opening the Sylvian fissure, STA- MCA bypass was performed by meticulous anastomosis of the parietal branch of the STA to the temporal cortical branch of the MCA. The Sylvian fissure was widely opened, and the temporal tip was sufficiently retracted posteriorly to visualize the retro-carotid space. The M1, ICA, A1, anterior choroidal artery (AChoA), posterior communicating artery (PcomA), and ICAbif aneurysm protruding into the frontal lobe were identified.
Critical steps: Control of aneurysm pressure and clipping
In Case 1, after temporary occlusion of the cervical ICA, A1, and M1, the aneurysm was dissected from the frontal lobe. After confirming both sides of the aneurysm neck, the permanent clip was applied carefully. However, the aneurysm dome near the neck was torn during the closing of the clip blades. Fortunately, the aneurysm was successfully clipped after deflating the aneurysm by suction from the torn hole. This inadvertent tearing of the aneurysm indicated that temporary occlusion of only the ICA, A1, and M1 was not the optimal strategy in treating very large and giant aneurysms for the following reasons: adequate aneurysm decompression was not obtained because of the residual inflow from the PcomA, AChoA, and ophthalmic artery, and the broad neck of very large and giant aneurysms could be damaged by excessive stress when closing the blade without adequate aneurysm decompression resulting in collapse.
In Case 2, to prevent inadvertent tearing of the aneurysm, almost complete trapping was achieved by clamping not only the cervical ICA, A1, M1, and PcomA, and making an intentional small incision in the aneurysm dome, performed before clipping the aneurysm. Suction from the incision ensured that the aneurysm was totally deflated. These critical steps ensured that the aneurysm was deflated, dissected safely and certainly from the surrounding structures, and finally successfully clipped. Patency of all vessels was confirmed by indocyanine green video-angiography in both cases after clipping of the aneurysm [
POSTOPERATIVE COURSE
No new postoperative deficits occurred in either patient. Diffusion-weighted imaging detected no new infarction on postoperative day 1 [
Case 1 was followed up in the outpatient hospital without neurological events for 3 years. Case 2 underwent ventriculoperitoneal shunt surgery for secondary hydrocephalus after subarachnoid hemorrhage. The patient’s preoperative symptoms, right hemiparesis, and aphasia, gradually improved with rehabilitation, and independence in activities of daily living, equivalent to modified Rankin scale 1, were achieved in 6 months.
DISCUSSION
Treatment for very large and giant intracranial aneurysms remains challenging for most neurosurgeons. Endovascular coiling is known to be effective, but satisfactory results are not guaranteed for very large and giant aneurysms. The complete obliteration rate of such aneurysms is lower, and the recanalization rate is higher than for small aneurysms.[
Some cases of giant aneurysms have been successfully treated by the high-flow extracranial-intracranial bypass,[
Numerous previous series have emphasized that crucial operative step in direct clipping of ICAbif aneurysms is dissecting the aneurysms and preserving the surrounding perforators, such as the AChoA, lateral lenticulostriate artery (LSA), PcomA perforators, and A1 perforators. Since the importance of careful dissection of perforators away from the aneurysm wall was described,[
The reduction of blood flow and pressure in the aneurysm by temporary occlusion of arteries is an essential concept for safe and sufficient dissection. Aneurysm dissection can proceed without temporary occlusion of parent arteries in small or medium-sized aneurysms with a relatively free dome,[
Trapping duration was 3 min, and no ischemic complications appeared in our Case 2. Although the acceptable time of clamping is controversial and only STA-MCA bypass cannot absolutely prevent ischemia, this assistant bypass is supposed to be an effective strategy for direct clipping in giant ICAbif aneurysms. However, this revascularization is not sufficient for the AChoA, which originates near the ICA top, and the ACA territory. In our two cases, the collateral flow to the ipsilateral ACA from the contralateral side through the anterior communicating artery was good, and both ACA areas were perfused from contralateral side during temporary occlusion of parent arteries. Even with STA-MCA bypass, the acceptable time for temporary occlusion of the arteries is controversial, and subsequent steps must be promptly performed.
After our experience with Case 1, we realized that more thorough control of aneurysm pressure than trapping was needed in cases of very large and giant aneurysms. Gentle traction on the clip may be useful to see the adjacent perforators behind the aneurysm,[
CONCLUSION
Trapping, deliberate aneurysm dome puncture, and suction decompression from the incision in conjunction with assistant STA-MCA bypass can achieve complete aneurysm deflation, and these techniques enable safe dissection of the aneurysm and direct clipping of the aneurysm neck. Direct clipping with this technique for very large and giant ICAbif aneurysms may be the optimal treatment choice with acceptable outcome if endovascular treatment remains suboptimal.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
www.surgicalneurologyint.com
References
1. Cantore G, Santoro A, Guidetti G, Delfinis CP, Colonnese C, Passacantilli E. Surgical treatment of giant intracranial aneurysms: Current viewpoint. Neurosurgery. 2008. 63: 279-89
2. Da Pian R, Pasqualin A, Scienza R. Direct microsurgical approach to aneurysms of the internal carotid bifurcation. Surg Neurol. 1980. 13: 27-37
3. Goddard AJ, Annesley-Williams D, Gholkar A. Endovascular management of unruptured intracranial aneurysms: Does outcome justify treatment?. J Neurol Neurosurg Psychiatry. 2002. 72: 485-90
4. Gonzalez NR, Duckwiler G, Jahan R, Murayama Y, Viñuela F. Challenges in the endovascular treatment of giant intracranial aneurysms. Neurosurgery. 2008. 62: 1324-35
5. Gupta SK, Khosla VK, Chhabra R, Mohindra S, Bapuraj JR, Khandelwal N. Internal carotid artery bifurcation aneurysms: Surgical experience. Neurol Med Chir (Tokyo). 2007. 47: 153-7
6. Inoue T, Tsutsumi K, Ohno H, Shinozaki M. Revascularization of the anterior cerebral artery with an A3-A3 anastomosis and a superficial temporal artery bypass using an A3-radial artery graft to trap a giant anterior communicating artery aneurysm: Technical case report. Neurosurgery. 2005. 57: E207-
7. Kalani MY, Wanebo JE, Martirosyan NL, Nakaji P, Zabramski JM, Spetzler RF. A raised bar for aneurysm surgery in the endovascular era. J Neurosurg. 2017. 126: 1731-9
8. Konczalla J, Platz J, Brawanski N, Güresir E, Lescher S, Senft C. Endovascular and surgical treatment of internal carotid bifurcation aneurysms: Comparison of results, outcome, and mid-term follow-up. Neurosurgery. 2015. 76: 540-50
9. Laranjeira M, Sadasivan B, Ausman JI. Direct surgery for carotid bifurcation artery aneurysms. Surg Neurol. 1990. 34: 250-4
10. Lee KC, Joo JY, Lee KS, Shin YS. Recanalization of completely thrombosed giant aneurysm: Case report. Surg Neurol. 1999. 51: 94-8
11. Lehecka M, Dashti R, Romani R, Celik O, Navratil O, Kivipelto L. Microneurosurgical management of internal carotid artery bifurcation aneurysms. Surg Neurol. 2009. 71: 649-67
12. Miyazawa N, Nukui H, Horikoshi T, Yagishita T, Sugita M, Kanemaru K. Surgical management of aneurysms of the bifurcation of the internal carotid artery. Clin Neurol Neurosurg. 2002. 104: 103-14
13. Mizoi K, Yoshimoto T, Takahashi A, Nagamine Y. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: Technical case report. Neurosurgery. 1996. 39: 165-8
14. Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with guglielmi detachable coils. Report of two cases with autopsy correlation. J Neurosurg. 1995. 83: 129-32
15. Morales-Valero SF, Brinjikji W, Murad MH, Wald JT, Lanzino G. Endovascular treatment of internal carotid artery bifurcation aneurysms: A single-center experience and a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2014. 35: 1948-53
16. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003. 98: 959-66
17. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001. 32: 1998-2004
18. Santoro A, Guidetti G, Dazzi M, Cantore G. Long saphenous-vein grafts for extracranial and intracranial internal carotid aneurysms amenable neither to clipping nor to endovascular treatment. J Neurosurg Sci. 1999. 43: 237-50
19. Savardekar AR, Patra DP, Narayan V, Bollam P, Guthikonda B, Nanda A. Internal carotid artery bifurcation aneurysms: Microsurgical strategies and operative nuances for different aneurysmal directions. Oper Neurosurg (Hagerstown). 2018. 15: 386-94
20. Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: Techniques and outcomes for 17 patients. Neurosurgery. 2001. 49: 646-58
21. Sekhar LN, Sen CN, Jho HD. Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg. 1990. 72: 35-41
22. Sengupta RP, Lassman LP, de Moraes AA, Garvan N. Treatment of internal carotid bifurcation aneurysms by direct surgery. J Neurosurg. 1975. 43: 343-51
23. Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of guglielmi detachable coils. Neurosurgery. 2002. 50: 239-49
24. van Rooij WJ, Sluzewski M. Endovascular treatment of large and giant aneurysms. AJNR Am J Neuroradiol. 2009. 30: 12-8
25. van Rooij WJ, Sluzewski M, Beute GN. Internal carotid bifurcation aneurysms: Frequency, angiographic anatomy and results of coiling in 50 aneurysms. Neuroradiology. 2008. 50: 583-7
26. Yaşargil MG, Boehm WB, Ho RE. Microsurgical treatment of cerebral aneurysms at the bifurcation of the internal carotid artery. Acta Neurochir (Wien). 1978. 41: 61-72
27. Zhang YJ, Barrow DL, Day AL. Extracranial-intracranial vein graft bypass for giant intracranial aneurysm surgery for pediatric patients: Two technical case reports. Neurosurgery. 2002. 50: 663-8