- Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan.
Correspondence Address:
Soichi Oya, Department of Neurosurgery, Saitama Medical Center, Saitama Medical University, Saitama, Japan.
DOI:10.25259/SNI_555_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shunya Hanakita, Masamichi Endo, Akira Saito, Soichi Oya. Trigeminal neuralgia due to intracranial venous reflux following central venous disease in a patient on hemodialysis: A case report. 16-Sep-2022;13:419
How to cite this URL: Shunya Hanakita, Masamichi Endo, Akira Saito, Soichi Oya. Trigeminal neuralgia due to intracranial venous reflux following central venous disease in a patient on hemodialysis: A case report. 16-Sep-2022;13:419. Available from: https://surgicalneurologyint.com/surgicalint-articles/11872/
Abstract
Background: A wide variety of conditions can cause trigeminal neuralgia (TN).
Case Description: We describe a rare case of a 77-year-old female patient on hemodialysis presenting with severe TN on the right side of the face for several weeks. She underwent multiple revisions using catheter for brachiocephalic venous stenosis over 6 years after a therapeutic arteriovenous fistula (AVF) was created in the left forearm. Her facial pain was consistent with Type 1 TN and remained intractable even after carbamazepine treatment. The initial magnetic resonance imaging did not demonstrate arterial compression on the right trigeminal nerve; instead, the vein adjacent to the right trigeminal nerve showed a hyperintense signal. In addition, the contralateral cortical veins and transverse sigmoid sinus were dilated. Angiography from the left brachial artery revealed intracranial venous reflux (IVR) through the left jugular vein due to an occluded brachiocephalic vein. Her pain was relieved immediately after her left upper arm was compressed with a sphygmomanometer to decrease the shunt. Surgical elimination of the AVF on the left forearm resulted in complete resolution of TN. Postoperative radiological examination revealed the resolution of IVR, and her TN has not recurred by her 6-month follow-up.
Conclusion: The radiological diagnosis of IVR might be complicated because the true causative lesion for focal neurological symptoms might be remotely located. IVR following central venous disease should be a differential when patients on hemodialysis present neurological symptoms.
Keywords: Central venous disease, Hemodialysis, Intracranial venous reflux, Trigeminal neuralgia
INTRODUCTION
A wide variety of conditions can cause trigeminal neuralgia (TN). Arterial compression accounts for 80% of all vascular compression observed in patients with Type 1 TN, which is characterized by the following: electric shock pain, trigger point, and a certain degree of carbamazepine effect.[
CASE DESCRIPTION
Patient history and examinations
A 77-year-old woman presented with a 3-week history of severe right-sided facial pain. The patient’s pain was characterized as electric shock-like pain, lasting 1–2 s, and triggered by touching the face. She had a 6-year history of end-stage renal disease managed by hemodialysis using an arteriovenous fistula (AVF) in her left forearm. Recently, the AVF in the left forearm required multiple percutaneous transluminal angioplasty (PTA) procedures because of intractable stenosis within the AVF. Stenosis at the brachiocephalic vein was also detected and was treated with PTA. One month before visiting our hospital, another AVF was newly placed in her right upper arm. She presented with no other neurological complaints apart from facial pain. Moreover, she had initially received carbamazepine with no relief of pain.
Although the characteristics of her facial pain were consistent with typical Type 1 TN, magnetic resonance imaging (MRI) indicated no apparent arterial abnormalities near the right trigeminal nerve [
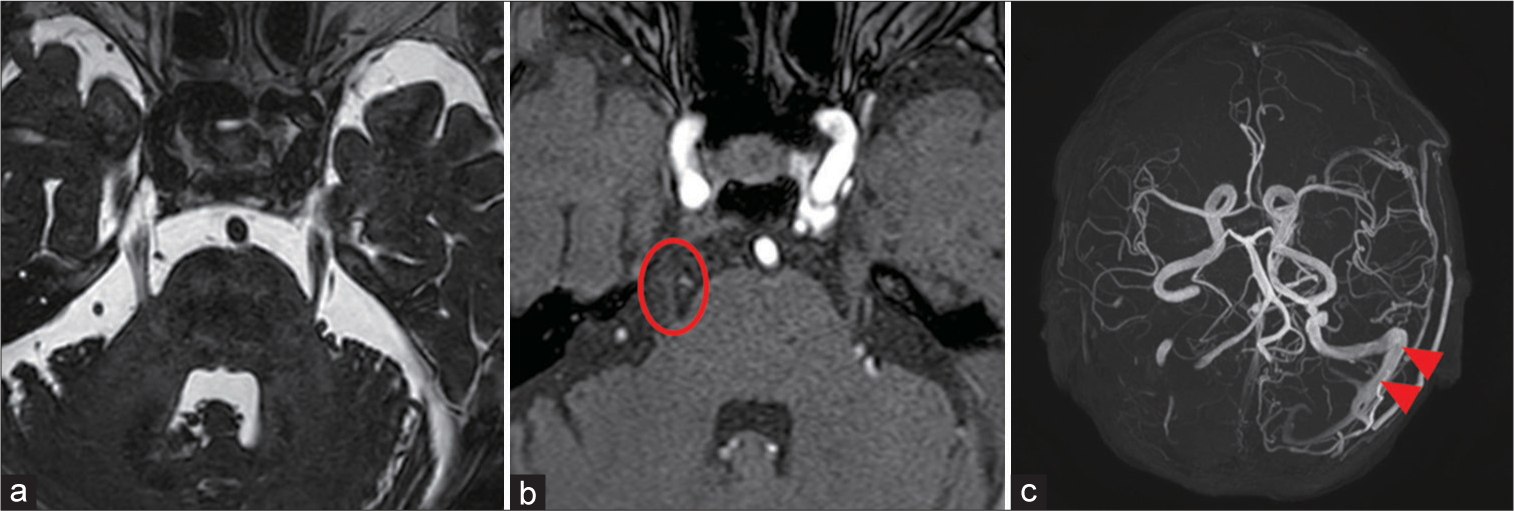
Figure 1:
(a-c) Preoperative magnetic resonance (MR) imaging. (a): An MR constructive interference in steady state image showed no arterial compression on the right trigeminal nerve. (b) A time-of-flight MR angiography demonstrated a slightly abnormal high-intensity signal from a vein adjacent to the right trigeminal nerve (red circle). (c) A maximum-intensity projection MR angiography revealed a retrograde venous flow in the left transverse and sigmoid sinus (red arrowhead).
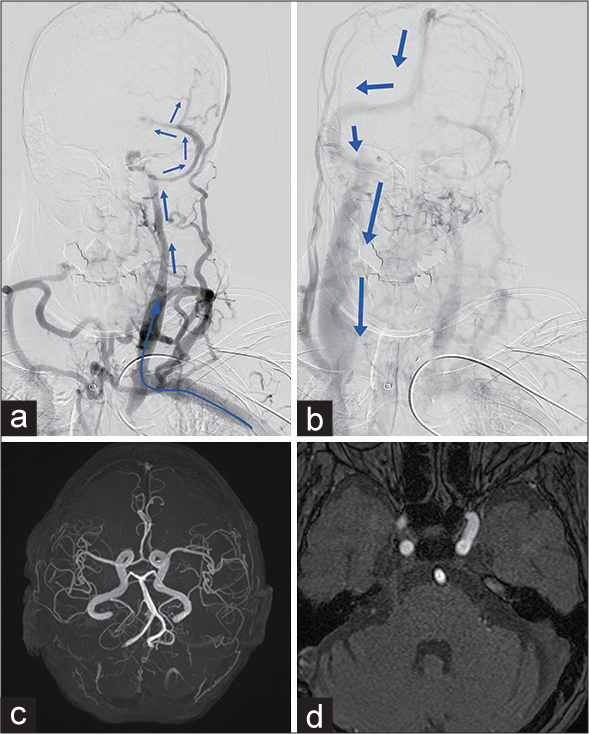
Figure 2:
(a) A preoperative angiography from the left brachial artery revealed an occlusion of the brachiocephalic vein and retrograde flow to the cranial venous system through the left jugular vein and lateral sinus (blue arrows), causing dilated cortical and diplopic veins. (b) The late phase of the angiography showed that the flow ultimately drained through the right jugular vein (blue arrows). (c) A postoperative maximumintensity projection magnetic resonance (MR) angiography demonstrated the disappearance of the retrograde venous flow in the left transverse-sigmoid sinus. (d) The abnormal high-intensity signal adjacent to the right trigeminal nerve on the time-of-flight MR angiography was reduced.
The postoperative course was uneventful, and her TN resolved immediately. Postoperative MRI showed the resolution of both the IVR [
DISCUSSION
The present case indicates that it is essential to search not only for the subtle findings around the trigeminal nerve but also the entire head-and-neck region for rare causes of TN, especially in the absence of arterial compression. In this case, IVR due to CVD after the therapeutic AVF in the left forearm appears to have caused dilatation and increased vessel pressure in the transverse pontine vein adjacent to the trigeminal nerve. The fact that her facial pain immediately resolved after a simple bed-side maneuver to compress the left forearm using a sphygmomanometer and that an abnormal signal on TOF-MRA disappeared after surgical closure of the shunt supported our speculation.
CVD is characterized by either stenosis or occlusion of veins and is a well-known chronic pathological condition in patients on dialysis.[
CONCLUSION
Patients on hemodialysis often present with the central venous stenosis or occlusion after the placement of multiple central venous catheters during their long treatment period. We emphasize the importance of considering the involvement of the central venous reflux when local imaging findings are lacking or inconsistent with TN with arterial compression in patients on hemodialysis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal AK, Patel BM, Haddad NJ. Central vein stenosis: A nephrologist’s perspective. Semin Dial. 2007. 20: 53-62
2. Baldauf J, Refaee EE, Marx S, Matthes M, Fleck S, Schroeder HW. Purely venous compression in trigeminal neuralgia-can we predict the outcome of surgery. Acta Neurochir (Wien). 2022. 164: 1567-73
3. Brown EC, Texakalidis P, Stedelin B, Tora MS, Rindler RS, Grossberg JA. Dural arteriovenous fistula presenting as trigeminal neuralgia: Two case reports and review of the literature. World Neurosurg. 2020. 139: 298-308
4. Caiza-Zambrano F, Palacio CM, Garbugino S, Gonzalez FM, Biolcati MB, Saucedo MÁ. Central venous reflux, a rare cause of neurological manifestations in hemodialysis patients: A case report and literature review. Neurointervention. 2022. 17: 58-64
5. Haruma J, Escalard S, Smajda S, Piotin M. Left temporal hemorrhage caused by cerebral venous reflux of a brachiobrachial hemodialysis fistula. Neuroradiology. 2020. 62: 1341-4
6. Herzig DW, Stemer AB, Bell RS, Liu AH, Armonda RA, Bank WO. Neurological sequelae from brachiocephalic vein stenosis: Report of 2 cases. J Neurosurg. 2013. 118: 1058-62
7. Iguchi T, Harada M, Kurihara S, Ichikawa T, Satoh S, Kobayashi M. Neurological symptoms due to intracranial venous congestion in a hemodialysis patient with arteriovenous shunted flow. Kidney Int Rep. 2020. 5: 2097-101
8. Jang J, Kim BS, Kim BY, Choi HS, Jung SL, Ahn KJ. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuroradiology. 2013. 55: 1205-11
9. Kim CH, Kang J, Choi DS, Park JH. Intracranial venous reflux caused by occlusion of the brachiocephalic vein mimicking dural arteriovenous fistula. World Neurosurg. 2018. 120: 438-41
10. Kundu S. Central venous disease in hemodialysis patients: Prevalence, etiology and treatment. J Vasc Access. 2010. 11: 1-7
11. Lumsden AB, MacDonald MJ, Isiklar H, Martin LG, Kikeri D, Harker LA. Central venous stenosis in the hemodialysis patient: Incidence and efficacy of endovascular treatment. Cardiovasc Surg. 1997. 5: 504-9
12. Miller JP, Acar F, Burchiel KJ. Classification of trigeminal neuralgia: Clinical, therapeutic, and prognostic implications in a series of 144 patients undergoing microvascular decompression. J Neurosurg. 2009. 111: 1231-4
13. Samaniego EA, Abrams KJ, Dabus G, Starr R, Linfante I. Severe venous congestive encephalopathy secondary to a dialysis arteriovenous graft. J Neurointerv Surg. 2013. 5: e37
14. Wang J, Niu H, Zhao K, Shu K, Lei T. Comparative analysis of trigeminal neuralgia caused by sole arterial and venous compression: Clinical features and surgical outcomes from 222 cases. Front Neurol. 2021. 12: 634945