- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
Correspondence Address:
Oscar Rubén ContrerasVázquez, Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Mexico City, Mexico.
DOI:10.25259/SNI_925_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rogelio Revuelta-Gutiérrez, Oscar Rubén Contreras-Vázquez, Fernando Piñón-Jiménez, Jaime Jesús Martínez-Anda. Trigeminal neuralgia secondary to epidermoid cyst and neurovascular conflict: An illustrative case with literature review. 09-Feb-2024;15:36
How to cite this URL: Rogelio Revuelta-Gutiérrez, Oscar Rubén Contreras-Vázquez, Fernando Piñón-Jiménez, Jaime Jesús Martínez-Anda. Trigeminal neuralgia secondary to epidermoid cyst and neurovascular conflict: An illustrative case with literature review. 09-Feb-2024;15:36. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12739
Abstract
Background: Trigeminal neuralgia (TN) is a highly disabling facial pain syndrome, historically known as the suicide disease, in which most cases can be cured with appropriate surgical treatment.
Case Description: We present the case of a 43-year-old male farmer with acute, self-limiting episodes of shock-like pain on the left side of the face that started in June of 2021. He was diagnosed with TN and was treated with carbamazepine. Magnetic resonance imaging was performed, which revealed an epidermoid cyst (EC) at the prepontine cistern with an extension to the left cerebellopontine angle. The neurosurgery department at our institution was consulted, which performed surgical tumor resection and Vth cranial nerve decompression. During the resection, a neurovascular conflict (NVC) was identified at the root entry zone. After the resection around the nerve and its whole tract was completed, a microvascular decompression (MVD) was performed.
Conclusion: TN secondary to EC in association with a NVC is a rare phenomenon, due to the growth pattern of the EC. TN may remit if an appropriate treatment is carried out. In cases of NVC, an MVD is required apart from an appropriate resection to achieve pain relief.
Keywords: Epidermoid cyst, Microvascular decompression, Neurovascular conflict, Trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) is a facial pain syndrome characterized by episodes of intense, lancinating facial pain followed by a period of relief. Between episodes, patients live in fear and anticipation of the next episode, coming to be known as the “suicide disease.”[
TN is classified based on the etiology as classical, secondary, and idiopathic.[
Epidermoid cysts (ECs), also called intracranial cholesteatomas, are rare lesions accounting for ≤1–2% of all intracranial tumors and 5–7% of cerebellopontine angle (CPA) lesions, just after schwannomas and meningiomas. Their primary location is in the CPA, followed by the parasellar region.[
ILLUSTRATIVE CASE
We present the case of a 43-year-old male farmer who presents acute, self-limiting episodes of shock-like pain located at the left maxillary branch territory of the trigeminal nerve. The episodes were triggered by chewing, touching the affected area, tooth brushing, and sometimes talking, with an intensity of 10/10, causing the patient to interrupt his daily activities. Initially, he was diagnosed with TN and treated with carbamazepine, 200 mg every eight h, and no magnetic resonance imaging (MRI) was taken. As the disease progressed, his pain became constant and disabling, and even though the patient had good therapeutic attachment, he increased the dose by his own accord, reaching toxic doses (5 tablets [200 mg] 5 times a day) because the pain was insufferable.
Study description
The patient eventually sought a second opinion, this time getting an MRI, where a tumoral lesion with EC characteristics in the CPA was identified, given an II in Revuelta’s Classification [
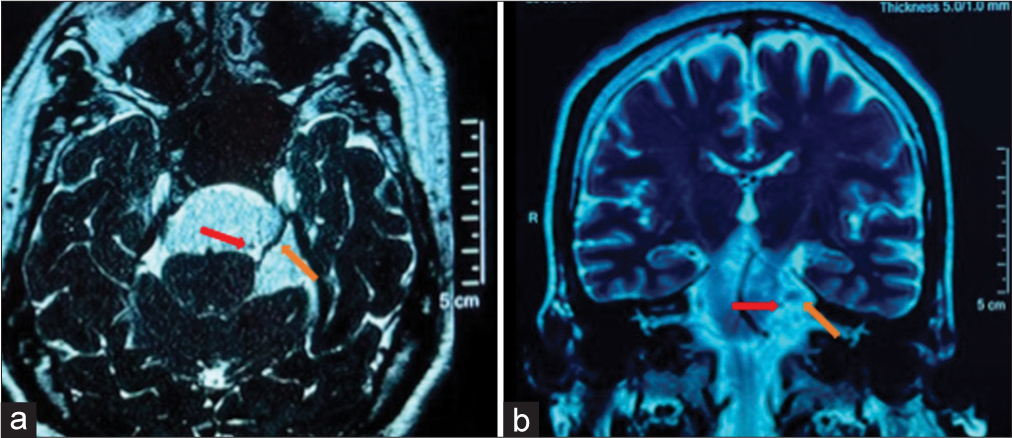
Figure 1:
Pre-surgical MRI. Compression of the pons by the EC and the immersion of the Vth CN in the tumor can be appreciated, as well as it being displaced superolaterally. (a) 3D FIESTA: The Vth CN adopted an S shape, and near the REZ, a hyperintense round figure that corresponds to the loop formed by the SCA (red arrow) can be observed. (b) T2: We appreciate a hypointense round which correspond to the SCA (red arrow) as the OV. Laterally, an oval figure that corresponds to the REZ of the Vth CN is seen. CN: Cranial nerve (orange arrow), EC: Epidermoid cyst, MRI: Magnetic resonance imaging, OV: Offending vessel, REZ: Root entry zone, SCA: Superior cerebellar artery (red arrow), 3D FIESTA: Three-dimensional fast imaging employing steady-state acquisition.
Due to the diagnosis and the excruciating pain, the patient came to the ER of our institution, where the pain was controlled, and the neurosurgery department was consulted.
The neurosurgery department decided to operate on him as a proprietary case based on the patient’s characteristics, doing so two weeks after the initial visit. No other focal deficits or pathologies were present in the patient before the surgery.
Surgical technique
The surgery was performed by Rogelio Revuelta-Gutiérrez (RRG). An extended microasterional approach was taken, involving a 30 mm diameter craniectomy at the internal angle of the transverse and sigmoid sinus. Before opening the dura, the cerebellum was tense, mainly due to the tumor, even after medical intervention by the anesthesiology team. To alleviate some of the tension, the dural opening was started in the inferolateral aspect of the craniectomy, depleting the cisterna magna. Subsequently, the dural opening was extended upwards to reach and deplete the cerebellomedullary cistern. However, multiple adhesions between the cerebellum, cerebellar fossa, and petrous eminence made it difficult to access the cistern.
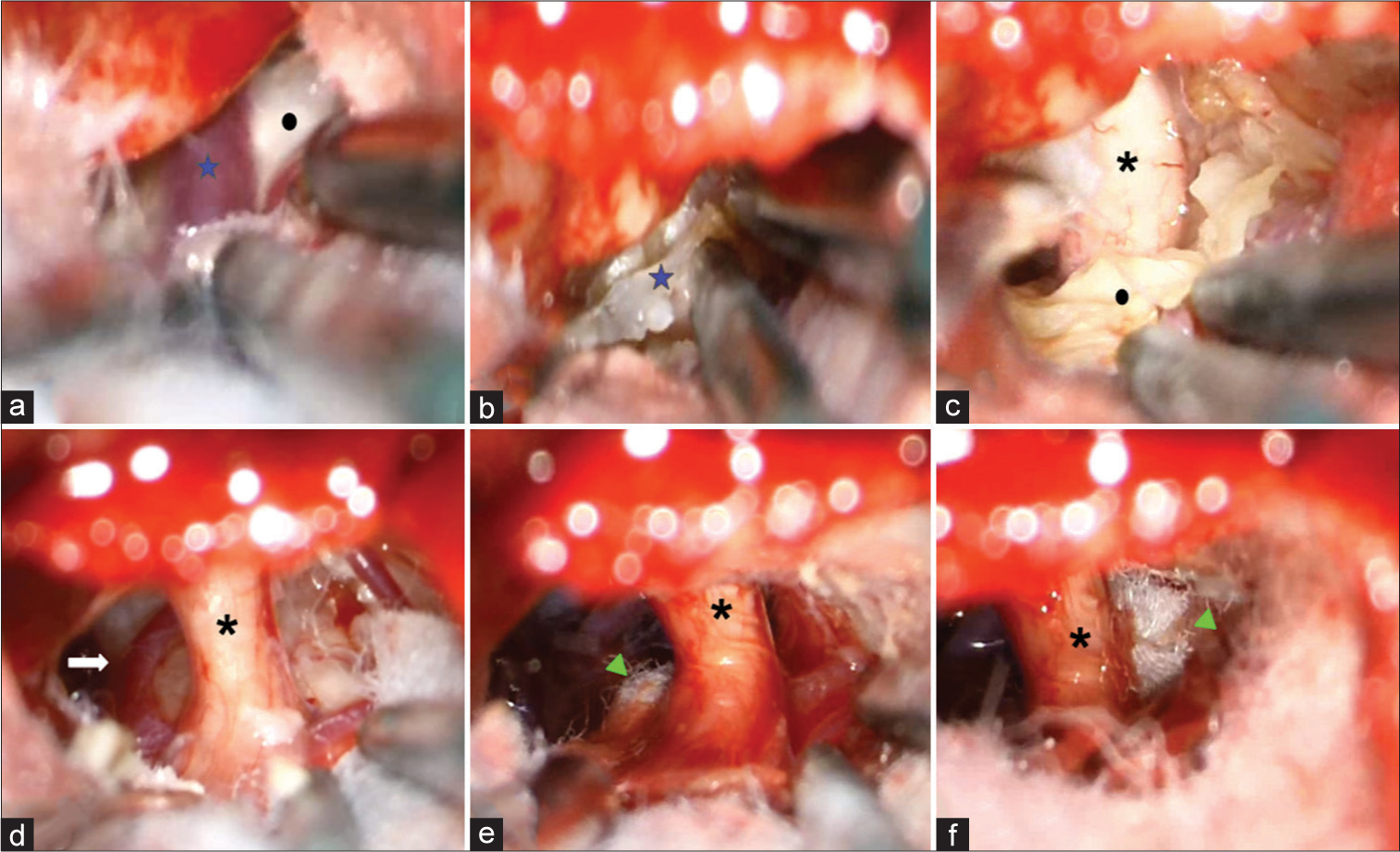
Once reached, the petrous vein was found to be displaced superficially [
Figure 2:
Intraoperative findings. (a) PV overlying the EC. (b) Coagulated PV. (c) Vth CN immersed in the EC. (d) After tumor debulking and capsular resection from the Vth CN, the SCA was exposed and didn’t relocate far from the REZ. (e) The MVD was started by placing a Teflon piece through the ventral part of the REZ. (f) MVD was completed by placing a Teflon piece through the dorsal aspect of the REZ. CN: Cranial nerve (asterisk), EC: Epidermoid cyst (circle), MVD: Microvascular decompression (arrowhead), PV: Petrous vein (star), REZ: Root entry zone, SCA: Superior cerebellar artery (arrow).
The first structure identified was the trigeminal nerve [
In the immediate postoperative period, the patient was pain-free, with a corneal reflex and no facial hypoesthesia. There were no postoperative cranial nerve (CN) deficits observed. Five days after surgery, the patient was cleared of any complications.
DISCUSSION
Clinically, primary and secondary TN are indistinguishable, but various studies have reported a statistically significant difference in the time of symptom onset, with secondary TN presenting in younger patients.[
Observations
The presence of NVC and EC is a rare phenomenon, as seen in our patient, has only been seen once in the experience of the main author RRG. The growth pattern of these tumors can explain this. EC tends to exhibit a slow and disseminated growth pattern, conforming to the shape of the subarachnoid space due to its soft and pliable nature before causing substantial compression with mass effect until all available space is occupied. This leads to a delayed diagnosis, with the presence of big and highly disseminated tumors with complex anatomy. This is a much more common phenomenon in other tumors that tend to displace the nerve, such as meningiomas and schwannomas, rather than compress or encase it.[
These anatomical variations have led to various classifications, depending on the author, based on the anatomical relationships between the tumor and the neurovascular structures.
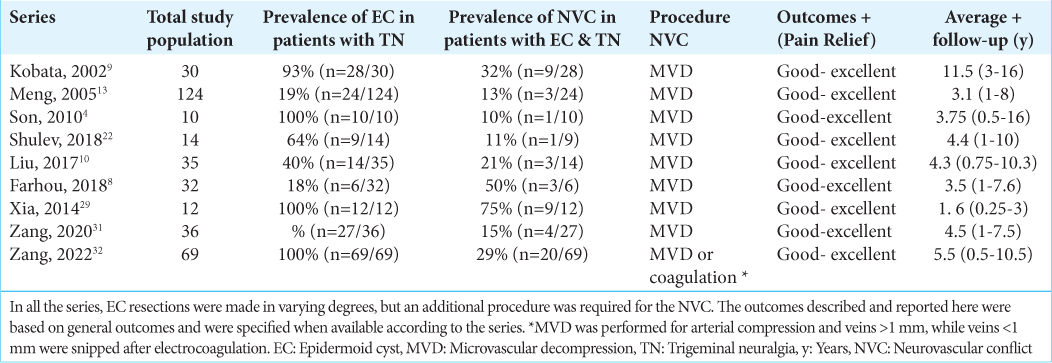
In
Not all of the series report which was the offending vessel (OV), but the most common arteries associated with NVC and EC were the anterior inferior cerebellar artery and superior cerebral artery, with some rarer cases reporting veins as the OV, especially the petrosal vein.[
This is especially important due to the pathogenesis of TN secondary to EC, and the MVD it’s a critical step for pain remission/surgical success. Some authors consider TN secondary to EC to be caused exclusively by an OV, but based on our experience and literature research, it seems to have a multifactorial etiology.[
Surgical considerations for NVC and EC
Surgery is currently the only curative therapy for EC. It offers the possibility to treat/alleviate the symptoms caused by the tumoral growth and, in many cases, can “cure” the patient or prevent regrowth.[
EC tends to remain asymptomatic for many years and presents with great extension at the time of diagnosis, representing a complex surgical challenge when total resection is desired, even for experimented hands. Open surgery offers many alternatives for the treatment of TN. In EC, the strategies taken along with tumor resection, which is the primary procedure, depend on the anatomical findings. In cases where salvage surgery is the only option, a radiosurgical procedure directed to the TN or other percutaneous procedures for pain relief can be an alternative.[
The surgical objective/approach varies between schools and surgeons; nonetheless, we should keep in mind what Bucy cleverly described: “It is important, however, that the wall of the cyst… which is the only living, growing part of the neoplasm, must be removed completely in order to prevent recurrence.”[
Many authors are in favor of an aggressive approach, as it may offer the patient a possible reintervention in a much longer period, reduce surgical comorbidity, and could even cause tumoral remission in some cases. We need to be conscious of the patient’s circumstances and expectations, the economic, human, and technical resources available, and the surgeon’s expertise.[
We consider that a moderate resection, focusing on symptomatic nerves, is the ideal surgery, looking for symptomatic remission (pain freedom), prevention of new deficits, and preservation of functionality, especially when the patient is previously completely functional, as in the case of our patient. This is the ideal approach for cases where the resources and circumstances are unfavorable but patient circumstances are optimal.
This focused resection with subtotal tumor removal is backed up by the linear growth hypothesis and the content rate of debris accumulation, especially because, even though the surgeon may have certainty of total capsular resection, sometimes it may be out of our hands. As the linear growth hypothesis postulates, some microscopic flakes may be left, and assuming that only a single cell remains after surgical resection, the patient would be at risk for recurrence at a period equal to his age at the time of the surgery plus nine months.[
For a focused surgery approach, a clear, wide pathway is made around the nerve with complete rectification of the neuroaxis and visualization of the nerve pathway before exiting through Meckel’s Cavum, along with a complete tumoral capsule resection from the affected nerve. Because, if left behind, TN may persist, as it has been seen in some series.[
As other authors have described [
Zhang Z et al. described their approach to intraoperative findings in a systematic way, which we consider a good alternative for systematization during surgery. They advocate for nerve combing in cases of severe invasion of the nerve with cholesteatomatous tissue, as a simple tumoral resection won’t alleviate pain.[
A helpful aid in the previously mentioned cases is the endoscope, which can eliminate blind spots during tumor removal and can help spot hidden NVC. Endoscopic assistance maximizes illumination and magnification around the tip of the endoscope and with this, allows visualization of corners and microsurgical blind spots. The caveat of this tool is the potential risk of damaging delicate structures in the posterior fossa due to new blind spots found behind the rod tip. In the hands of experienced surgeons, this risk can be minimal. This tool is useful but not necessary and should be used with caution so as not to damage vital structures during its use.[
CONCLUSION
We operate on patients, not images. The extent of tumor and capsular resection does not solely determine a successful surgery. Instead, our primary goal is to provide symptom relief, particularly in cases of EC where the growth is typically slow and benign. Considering this, a focused approach is reasonable, especially when dealing with patients who are only presenting TN. When addressing TN, it is crucial to perform EC resection alongside an intentional search for NVC, even though NVC is rare in EC cases, as alleviation of pain relies on identifying and addressing it. As the surgery progresses, alternative surgical strategies such as neuropraxia or nerve combing might be considered in specific situations, as well as the aid of an endoscope which can be useful. but not necessary, always prioritizing patient safety.
They are his nerves, not yours.
-Dr. Revuelta GR.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aboud E, Abolfotoh M, Pravdenkova S, Gokoglu A, Gokden M, Al-Mefty O. Giant intracranial epidermoids: Is total removal feasible?. J Neurosurg. 2015. 122: 743-56
2. Alvord EC. Growth rates of epidermoid tumors. Ann Neurol. 1977. 2: 367-70
3. Barker FG, Jannetta PJ, Babu RP, Pomonis S, Bissonette DJ, Jho HD. Long-term outcome after operation for trigeminal neuralgia in patients with posterior fossa tumors. J Neurosurg. 1996. 84: 818-25
4. Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Lacerda Leal PR, Nurmikko T. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020. 19: 784-96
5. Bucy PC. Intradiploic epidermoid (cholesteatoma) of the skull. Arch Surg. 1935. 31: 190-9
6. Bullitt E, Tew JM, Boyd J. Intracranial tumors in patients with facial pain. J Neurosurg. 1986. 64: 865-71
7. Cruccu G, Finnerup NB, Jensen TS, Scholz J, Sindou M, Svensson P. Trigeminal neuralgia: New classification and diagnostic grading for practice and research. Neurology. 2016. 87: 220-8
8. Farhoud A, Khedr W, Aboul-Enein H. Surgical resection of Cerebellopontine epidermoid cysts: Limitations and outcome. J Neurol Surg B Skull Base. 2018. 79: 167-72
9. Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: Pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002. 50: 276-86
10. Liu P, Liao C, Zhong W, Yang M, Li S, Zhang W. Symptomatic trigeminal neuralgia caused by cerebellopontine angle tumors. J Craniofac Surg. 2017. 28: e256-8
11. Martínez-Anda J, Barges-Coll J, Ponce-Gomez J, Perez-Pena N, Revuelta-Gutierrez R. Surgical management of trigeminal neuralgia in elderly patients using a small retrosigmoidal approach: Analysis of efficacy and safety. J Neurol Surg A Cent Eur Neurosurg. 2015. 76: 39-45
12. Martinez-Anda JJ, Suarez G, David P, Martríinez DP, Pradel RA, Javier E. Endoscopice assisted surgery for cerebello pontine angle pathology: Technical note and surgical results in a series of patients. Arch Neurosurg. 2021. 1: 35-47
13. Meng L, Yuguang L, Feng L, Wandong S, Shugan Z, Chengyuan W. Cerebellopontine angle epidermoids presenting with trigeminal neuralgia. J Clin Neurosci. 2005. 12: 784-6
14. Patel SK, Liu JK. Overview and history of trigeminal neuralgia. Neurosurg Clin N Am. 2016. 27: 265-76
15. Peciu-Florianu I, Régis J, Levivier M, Dedeciusova M, Reyns N, Tuleasca C. Trigeminal neuralgia secondary to meningiomas and vestibular schwannoma is improved after stereotactic radiosurgery: A systematic review and meta-analysis. Stereotact Funct Neurosurg. 2020. 99: 6-16
16. Pop MM, Bouros D, Klimko A, Florian IA, Florian IS. Intracranial epidermoid cysts: Benign entities with malignant behavior: Experience with 36 cases. Sci Rep. 2023. 13: 6474
17. Rappaport Z. Epidermoid tumour of the cerebellopontine angle as a cause of trigeminal neuralgia. Neurochirurgia (Stuttg). 1985. 28: 211-2
18. Revuelta-Gutiérrez R, Díaz-Romero RF, Vales-Hidalgo LO, Hinojosa-González R, Barges-Coll J. Cerebellopontine angle epidermoid cyst. Experience of 43 cases with long-term follow-up. Cir Ciruj. 2009. 77: 257-65
19. Revuelta-Gutiérrez R, López-González MA, Soto-Hernández JL. Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surg Neurol. 2006. 66: 32-6
20. Revuelta-Gutierrez R, Martinez-Anda JJ, Coll JB, CamposRomo A, Perez-Peña N. Efficacy and safety of root compression of trigeminal nerve for trigeminal neuralgia without evidence of vascular compression. World Neurosurg. 2013. 80: 385-9
21. Shear BM, Jin L, Zhang Y, David WB, Fomchenko EI, ErsonOmay EZ. Extent of resection of epidermoid tumors and risk of recurrence: Case report and meta-analysis. J Neurosurg. 2020. 133: 291-301
22. Shulev Y, Trashin A, Gordienko K. Secondary trigeminal neuralgia in cerebellopontine angle tumors. Skull Base. 2011. 21: 287-94
23. Singh R, Prasad RS, Singh A. Evaluation of cerebellopontine angle epidermoid presenting with cranial nerve deficit: A surgical perspective. Asian J Neurosurg. 2020. 15: 573-8
24. Son DW, Choi CH, Cha SH. Epidermoid tumors in the cerebellopontine angle presenting with trigeminal neuralgia. J Korean Neurosurg Soc. 2010. 47: 271-7
25. Tai AX, Nayar VV. Update on trigeminal neuralgia. Curr Treat Options Neurol. 2019. 21: 42
26. Vasquez JA, Fonnegra JR, Diez JC, Fonnegra A. Treatment of epidermoid tumors with gamma knife radiosurgery: Case series. Surg Neurol Int. 2016. 7: 116
27. Vernon V, Naik H, Guha A. Surgical management of cerebellopontine angle epidermoid cysts: An institutional experience of 10 years. Br J Neurosurg. 2022. 36: 203-12
28. Wakabayashi T, Tamaki N, Satoh H, Matsumoto S. Epidermoid tumor presenting as painful tic convulsif. Surg Neurol. 1983. 19: 244-6
29. Xia L, Zhong J, Zhu J, Wang YN, Dou NN, Liu MX. Cholesteatoma of cerebellopontine angle presented as trigeminal neuralgia. J Craniofac Surg. 2014. 25: 1540-2
30. Yaşargil MG, Abernathey CD, Sarioglu A. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989. 24: 561-7
31. Zhang Y, Yu F, Zhao Z, Men X, Shi W. Surgical treatment of secondary trigeminal neuralgia induced by cerebellopontine angle tumors: A single-center experience. World Neurosurg. 2020. 141: e508-13
32. Zhang Z, Wang W, Yu F, Kwok SC, Wang Y, Yin J. Strategies for intraoperative management of the trigeminal nerve and long-term follow-up outcomes in patients with trigeminal neuralgia secondary to an intracranial epidermoid cyst. Front Surg. 2022. 9: 930261