- Department of Neurosurgery, Baylor College of Medicine/Texas Children’s Hospital, Houston, Texas, United States,
- Department of Neurosurgery, Indiana University School of Medicine/Riley Children’s Hospital, Indianapolis, Indiana, United States,
- Department of Otolaryngology, Baylor College of Medicine/ Texas Children’s Hospital, Houston, Texas, United States,
- Department of Neurosurgery, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital, Chicago, Illinois, United States.
Correspondence Address:
Sandi Lam
Department of Neurosurgery, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital, Chicago, Illinois, United States.
DOI:10.25259/SNI_482_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brandon Emilio Bertot1, Melissa Lo Presti1, Katie Stormes1, Jeffrey S. Raskin2, Andrew Jea2, Daniel Chelius3, Sandi Lam4. Trigeminal schwannoma presenting with malocclusion: A case report and review of the literature. 08-Aug-2020;11:230
How to cite this URL: Brandon Emilio Bertot1, Melissa Lo Presti1, Katie Stormes1, Jeffrey S. Raskin2, Andrew Jea2, Daniel Chelius3, Sandi Lam4. Trigeminal schwannoma presenting with malocclusion: A case report and review of the literature. 08-Aug-2020;11:230. Available from: https://surgicalneurologyint.com/surgicalint-articles/10198/
Abstract
Background: Trigeminal schwannomas are rare tumors of the trigeminal nerve. Depending on the location, from which they arise along the trigeminal nerve, these tumors can present with a variety of symptoms that include, but are not limited to, changes in facial sensation, weakness of the masticatory muscles, and facial pain.
Case Description: We present a case of a 16-year-old boy with an atypical presentation of a large trigeminal schwannoma: painless malocclusion and unilateral masticatory weakness. This case is the first documented instance; to the best of our knowledge, in which a trigeminal schwannoma has led to underbite malocclusion; it is the 19th documented case of unilateral trigeminal motor neuropathy of any etiology. We discuss this case as a unique presentation of this pathology, and the relevant anatomy implicated in clinical examination aid in further understanding trigeminal nerve pathology.
Conclusion: We believe our patient’s underbite malocclusion occurred secondary to his trigeminal schwannoma, resulting in associated atrophy and weakness of the muscles innervated by the mandibular branch of the trigeminal nerve. Furthermore, understanding the trigeminal nerve anatomy is crucial in localizing lesions of the trigeminal nerve.
Keywords: Cranial neuropathy, Malocclusion, Pediatric neurosurgery, Skull base tumor, Trigeminal schwannoma
INTRODUCTION
Schwannomas of the trigeminal nerve are rare; they constitute about 0.07–0.3% of all intracranial tumors and 0.8–5% of intracranial schwannomas.[
Common presenting symptoms of trigeminal schwannomas include facial numbness (65.8%), headaches (58.9%), gait disturbance (42.5%), hearing deterioration (24.7%), diplopia (24.7%), facial pain (20.5%), visual deterioration (17.8%), pathological laughter (12.3%), proptosis (10.9%), limb weakness (9.6%), and seizures (4.1%).[
We present a case of a 16-year-old boy with an atypical presentation of a large trigeminal schwannoma: painless malocclusion and unilateral masticatory weakness. This case is the first documented instance; to the best of our knowledge, in which a trigeminal schwannoma has led to underbite malocclusion. We aimed to discuss this case as a unique presentation of this pathology and the key role understanding relevant anatomy holds in diagnosis and understanding of trigeminal nerve pathology.
CASE DESCRIPTION
A 16-year-old boy with a history of the right-sided Bell’s Palsy presented with complaints of a severe underbite and excessive drooling on the left side. These symptoms were progressive over several years to the point that the patient could not comfortably close his mouth. The patient had an unremarkable dental history with no previous oral trauma/procedures. On examination, the patient had facial asymmetry at rest, with the normal function of the facial nerve bilaterally. The facial sensation was intact symmetrically in the V1, V2, and V3 distributions to light touch. He had nystagmus on the right lateral gaze and mild weakness of the left masseter. There was no other indication of brainstem or cranial nerve dysfunction. The remainder of the physical examination was normal.
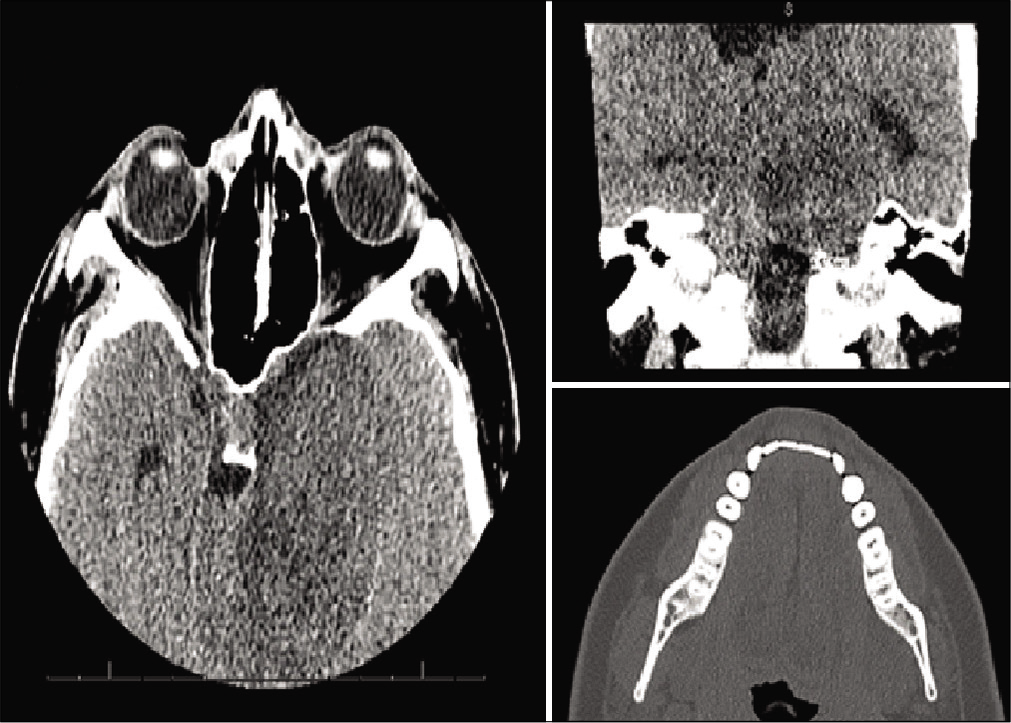
A computed tomography (CT) scan maxillofacial without contrast was performed, demonstrating a 5.2 × 6.6 × 4.3 cm intra-axial mass with compression and displacement of the brainstem [
Figure 1:
Diagnostic computed tomography maxillofacial images figure description: images a and b demonstrate axial and coronal views of a 5.2 × 6.6 × 4.3 cm intra-axial mass with compression and displacement of the brainstem. Image C demonstrates an axial view of the mandible, showing asymmetry of the left mandibular ramus and muscular hypotrophy on the left side.
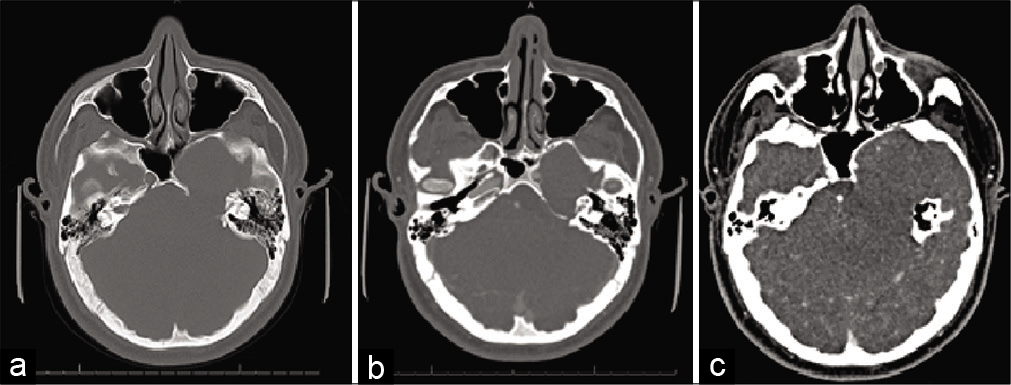
CT angiography (CTA) of the head and magnetic resonance imaging (MRI) brain with and without contrast was performed for further evaluation. CTA demonstrated bony remodeling of the left sphenoid and petrous portion of the temporal bones related to the mass with external compression (approximately 50% narrowing) of the cavernous and petrous segment of the left internal carotid artery (ICA) [
Figure 2:
Diagnostic computed tomography angiogram figure description: bony erosion and remodeling of the left sphenoid and petrous temporal bones are seen, related to the mass (a and b). On contrasted imaging (c), angiography demonstrates external compression (approximately 50% narrowing) of the cavernous and petrous segment of the left internal carotid artery.
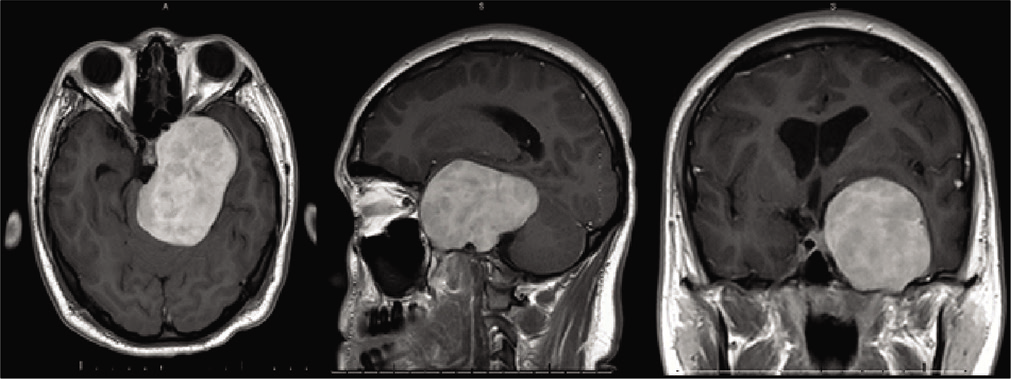
Figure 3:
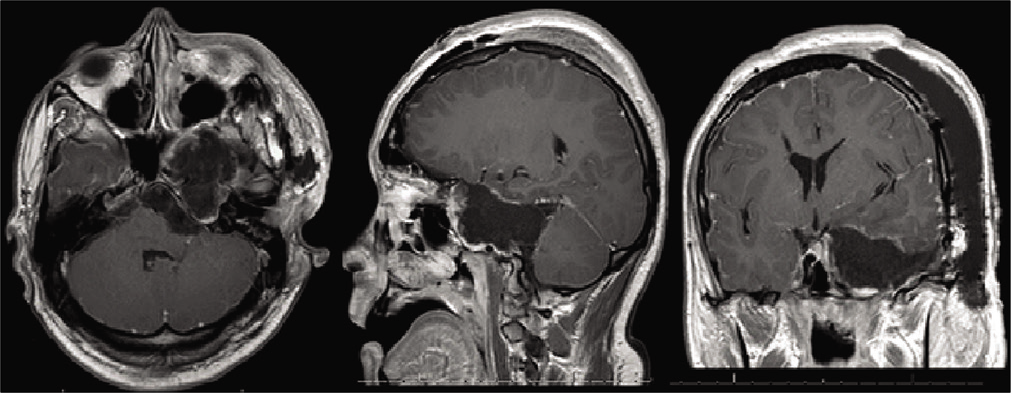
Preoperative magnetic resonance imaging figure description: contrasted T1-weighted magnetic resonance imaging with axial, sagittal, and coronal views of the 5.2 × 7.8 × 5.1 cm heterogeneously extra-axial enhancing mass centered in the left mesial temporal region with involvement of the skull base and left foramen ovale, rotundum and spinosum, encasement and moderate narrowing of the left internal carotid artery, mild obstructive hydrocephalus, and marked compression of the brainstem.
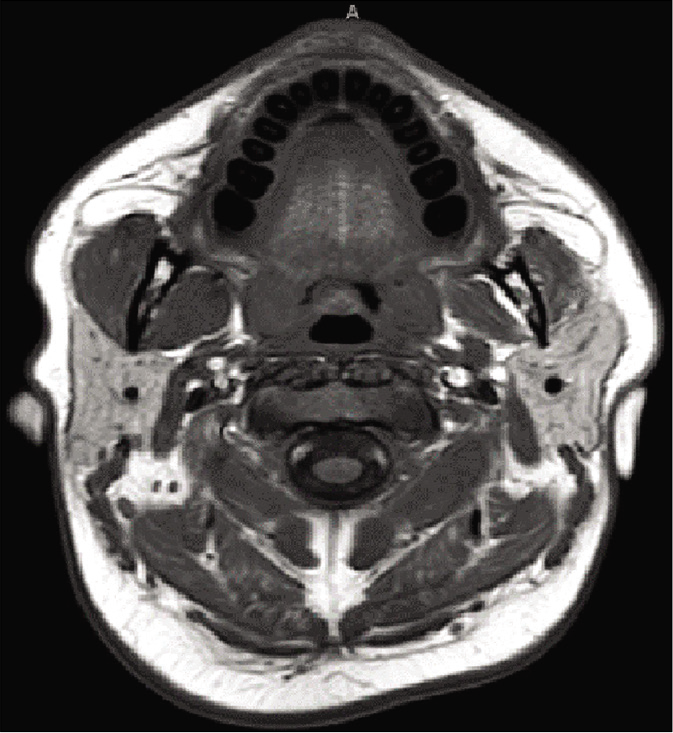
The patient underwent a left orbitozygomatic craniotomy for resection. Pathology of the resected tumor was consistent with the World Health Organization Grade I Schwannoma. Postoperative imaging demonstrated near-complete resection with a thin rim of the residual tumor along the lateral dural margin of the left cavernous sinus/lesser sphenoid wing and at the floor of the left middle cranial fossa extending posteriorly to the roof of the remodeled left petrous temporal bone [
Figure 5:
Postoperative magnetic resonance imaging figure description: contrasted T1-weighted magnetic resonance imaging with axial, sagittal, and coronal views demonstrates near complete resection with a thin rim of residual tumor along the lateral dural margin of the left cavernous sinus/lesser sphenoid wing and at the floor of the left middle cranial fossa extending posteriorly to the roof of the remodeled left petrous temporal bone.
DISCUSSION
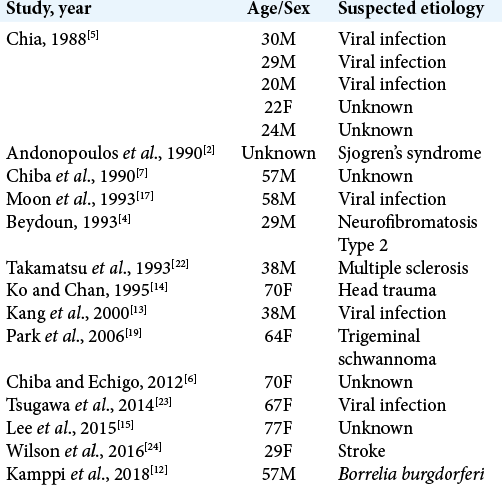
This is a unique case of a 16-year-old boy with an atypical presentation of a unilateral trigeminal motor neuropathy (UTMN) and severe underbite malocclusion attributable to a large trigeminal schwannoma. This patient experienced only weakness and atrophy of the muscles innervated by the mandibular branch of the trigeminal nerve, which likely resulted in his severe underbite malocclusion due to chronic compression of the trigeminal nerve in this location causing underdevelopment and muscle atrophy. This specific presentation of trigeminal nerve dysfunction is referred to as a pure UTMN. In fact, before this case, only 18 other cases of pure UTMN have been reported in the literature [
The clinical presentations of trigeminal schwannomas vary depending on the location of the trigeminal nerve, from which the tumor arises, the degree of extension into other areas of the brain, and the overall size of the tumor. Clinical suspicion for trigeminal schwannomas should be raised in the presence of slowly progressive symptoms with a predominance of trigeminal nerve-related symptoms, including facial numbness and masticatory muscle wasting.[
Anatomy of the trigeminal nerve
Trigeminal nerve anatomy is complex but crucial in understanding the localization of symptomatology and pathology of related lesions. The trigeminal nerve is the largest of the 12 cranial nerves. It originates from the brainstem as four nuclei, three sensories, and one motor.[
In addition, the trigeminal nerve is commonly discussed by segments: brainstem, cisternal, Meckel’s cave, cavernous, and peripheral segments.[
Other systems were developed to further classify trigeminal schwannomas by location; Jefferson described Type A, B, or C trigeminal schwannomas, depending on their location in the middle, posterior, or both middle and posterior fossas, respectively.[
The mandibular division/V3 can be divided into three trunks: the undivided trunk, the anterior trunk, and the posterior trunk.[
CONCLUSION
This case demonstrates a unique presentation of an adolescent patient presenting with a trigeminal schwannoma manifesting with a pure UTMN, resulting in severe underbite malocclusion, which is the first documented instance of this etiology. Although most trigeminal schwannomas present with sensory loss and pain, our patient presented with a severe underbite as a result of the weakness of the muscles innervated by the mandibular branch of the trigeminal nerve. Due to a complex anatomy, trigeminal nerve tumors can present with a variety of symptoms. Understanding relevant anatomy is key to localizing pathology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal A. Intracranial trigeminal schwannoma. Neuroradiol J. 2015. 28: 35-41
2. Andonopoulos A, Lagos G, Drosos A, Moutsopoulos H. The spectrum of neurological involvement in Sjögren’s syndrome. Br J Rheumatol. 1990. 29: 21-4
3. Bathla G, Hegde A. The trigeminal nerve: An illustrated review of its imaging anatomy and pathology. Clin Radiol. 2013. 68: 203-13
4. Beydoun SR. Unilateral trigeminal motor neuropathy as a presenting feature of neurofibromatosis Type 2 (NF2). Muscle Nerve. 1993. 16: 1136-7
5. Chia LG. Pure trigeminal motor neuropathy. Br Med J (Clin Res Ed). 1988. 296: 609-10
6. Chiba M, Echigo S. Unilateral atrophy of the masticatory muscles and mandibular ramus due to pure trigeminal motor neuropathy: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012. 113: e30-4
7. Chiba S, Motoi Y, Noro H, Asakura K, Matsumoto H. A case of pure trigeminal motor neuropathy. Rinsho Shinkeigaku. 1990. 30: 883-7
8. Fillmore EP, Seifert MF.editors. Anatomy of the trigeminal nerve. Nerves and Nerve Injuries. San Diego: Academic Press; 2015. p. 319-50
9. Glasauer FE, Tandon PN. Trigeminal neurinoma in adolescents. J Neurol Neurosurg Psychiatry. 1969. 32: 562-8
10. Goel A, Muzumdar D, Raman C. Trigeminal neuroma: Analysis of surgical experience with 73 cases. Neurosurgery. 2003. 52: 783-90
11. Jefferson G. The Trigeminal neurinomas with some remarks on malignant invasion of the gasserian ganglion. Neurosurgery. 1955. 1: 11-54
12. Kamppi A, Kämppi L, Kemppainen P, Kanerva M, Toppila J, Auranen M. Focal atrophy of the unilateral masticatory muscles caused by pure trigeminal motor neuropathy: Case report. Clin Case Rep. 2018. 6: 939-43
13. Kang Y, Lee E, Hwang M. Pure trigeminal motor neuropathy: A case report. Arch Phys Med Rehabil. 2000. 81: 995-8
14. Ko KF, Chan KL. A case of isolated pure trigeminal motor neuropathy. Clin Neurol Neurosurg. 1995. 97: 199-200
15. Lee D, Ha Y, Kim M, Yoo B. Pontine infarction presenting with isolated trigeminal motor neuropathy. J Neurol Sci. 2015. 357: e390
16. McCormick PC, Bello JA, Post KD. Trigeminal schwannoma. Surgical series of 14 cases with review of the literature. J Neurosurg. 1988. 69: 850-60
17. Moon JS, Park YK, Sunwoo IN. A case of pure trigeminal motor neuropathy. J Korean Neurol Assoc. 1993. 11: 136-7
18. Nager GT. Neurinomas of the trigeminal nerve. Am J Otolaryngol. 1984. 5: 301-33
19. Park KS, Chung J, Jeon BS, Park S, Lee K. Unilateral trigeminal mandibular motor neuropathy caused by tumor in the foramen ovale. J Clin Neurol. 2006. 2: 194-7
20. Samii M, Migliori MM, Tatagiba M, Babu R. Surgical treatment of trigeminal schwannomas. J Neurosurg. 1995. 82: 711-8
21. Sharma BS, Ahmad FU, Chandra PS, Mahapatra AK. Trigeminal schwannomas: Experience with 68 cases. J Clin Neurosci. 2008. 15: 738-43
22. Takamatsu K, Takizawa T, Miyamoto T. A case of pure trigeminal motor neuropathy. Rinsho Shinkeigaku. 1993. 33: 541-5
23. Tsugawa J, Ouma S, Fukae J, Tsuboi Y, Maki Y, Hokezu Y. Pure trigeminal motor neuropathy with an antecedent infection: A case report. Rinsho Shinkeigaku. 2014. 54: 515-7
24. Wilson M, Hodgson E, Felstead A. Focal atrophy of the masticatory muscles caused by pure trigeminal motor neuropathy: Case report. Br J Oral Maxillofac Surg. 2016. 54: e13-4