- Department of Neurology, Osaka Neurological Institute,
- Department of Clinical Neuroscience, Tokushima University,
- Departments of Neurosurgery, Osaka Neurological Institute, Toyonaka, Japan,
- Neuroradiology, Osaka Neurological Institute, Toyonaka, Japan.
Correspondence Address:
Shinichi Matsumoto, Department of Neurology, Osaka Neurological Institute, Toyonaka, Japan.
DOI:10.25259/SNI_173_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shinichi Matsumoto1, Yuki Yamamoto2, Koji Fujita2, Ryosuke Miyamoto2, Hidetaka Koizumi1, Akihiro Tateishi3, Naoaki Yamada4, Yuishin Izumi2. Truncal dystonia with isolated middle cerebral artery ischemia: A case report of revascularization therapy for dystonia. 15-Apr-2022;13:155
How to cite this URL: Shinichi Matsumoto1, Yuki Yamamoto2, Koji Fujita2, Ryosuke Miyamoto2, Hidetaka Koizumi1, Akihiro Tateishi3, Naoaki Yamada4, Yuishin Izumi2. Truncal dystonia with isolated middle cerebral artery ischemia: A case report of revascularization therapy for dystonia. 15-Apr-2022;13:155. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11528
Abstract
Background: Dystonia is a rare movement disorder with some cases being difficult to treat. Although dystonia can occur as a symptom of moyamoya disease, few studies have reported truncal dystonia occurring with middle cerebral artery (MCA) stenosis. Here, we report a case of truncal dystonia with MCA occlusion.
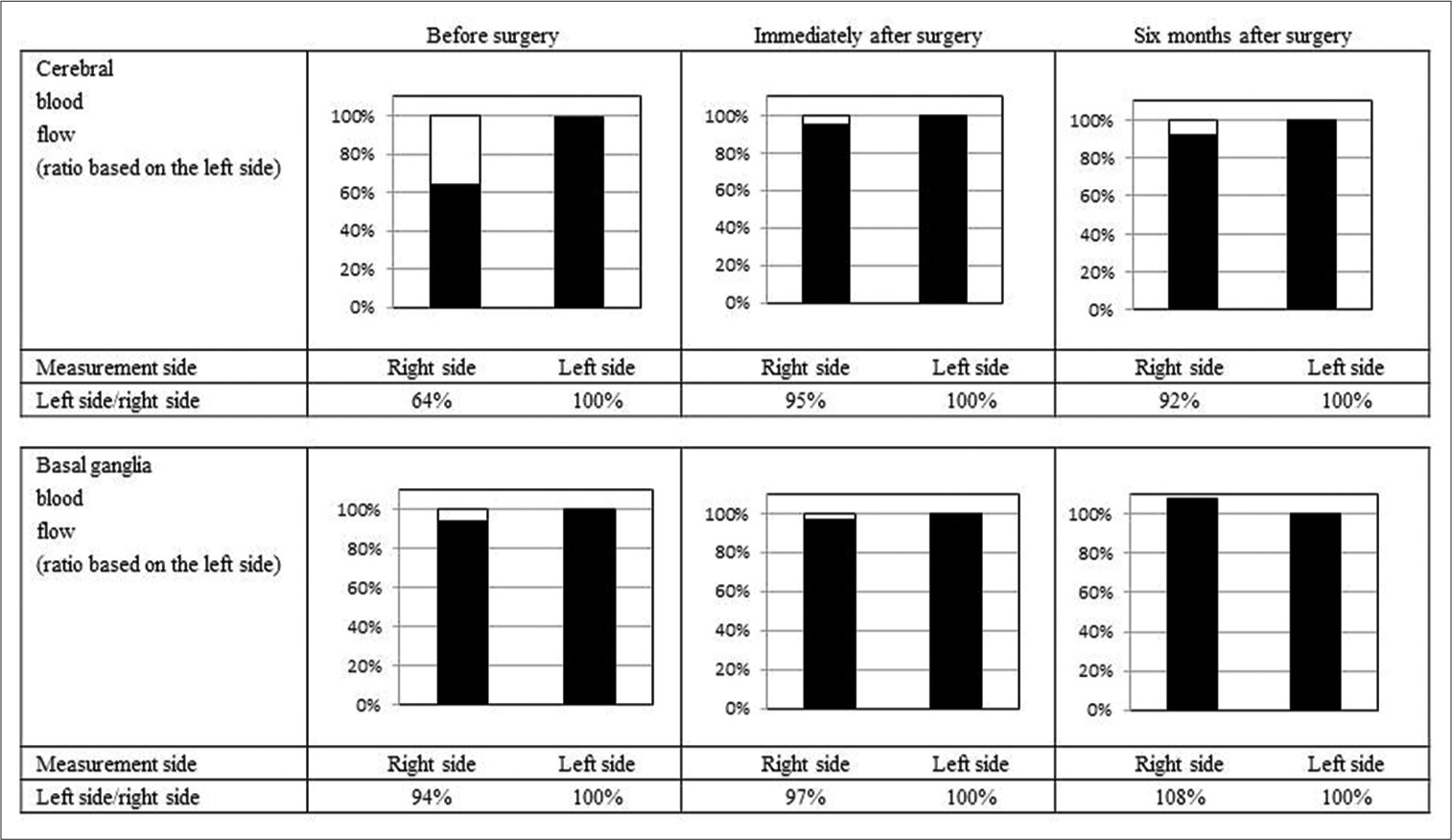
Case Description: The patient was a 48-year-old female clerical worker who lived alone. An abnormal cervical posture initially appeared 7 years before (right flexion). Symptoms improved with medication and botulinum toxin injection. Five years before this report, her symptoms worsened, so the dose of oral medication was increased and botulinum treatment was performed, but the symptoms did not improve. The patient showed decreased cerebral blood flow (CBF) in the cortical areas but not in the basal ganglia. We performed superficial temporal artery-MCA bypass surgery because we believed that the dystonia was due to right MCA stenosis. The patient’s symptoms improved immediately after surgery, except for her mild cervical backbend. Seven months after the surgery, the patient’s involuntary movements showed further improvement, and symptoms have not worsened even after 2 years.
Conclusion: Revascularization therapy improved CBF and truncal dystonia and could be a viable treatment option for dystonia with ischemia in the MCA region. Extensive cerebral ischemia can result in cortical inhibition loss or over-adapted cerebral plasticity and cause dystonia. Revascularization therapy may be useful for patients with dystonia and decreased CBF in the MCA region.
Keywords: Cerebral blood flow, Dystonia, Middle cerebral artery stenosis, Network pathophysiology model, Revascularization
INTRODUCTION
Dystonia is a clinical syndrome characterized by sustained muscle contractions that cause twisting, repetitive movements, or abnormal postures.[
Involuntary movements can be diagnosed based on clinical symptoms. However, there are various causes, and it may be difficult to select a treatment option. Involuntary movements, such as dystonia and ballism, occur as symptoms of moyamoya disease.[
CASE DESCRIPTION
The patient was a 48-year-old female clerical worker who lived alone. An abnormal cervical posture initially appeared 7 years before this study (right flexion). The treatment included the prescription of trihexyphenidyl hydrochloride (6 mg), clonazepam (1.5 mg), and baclofen (15 mg), which was effective and resulted in good outcomes. Botulinum treatment was also prescribed and effective, and the symptoms almost disappeared. Five years before this study, the abnormal cervical posture worsened, and symptoms of cervical retroflexion and trunk anteflexion developed. Doses were increased (trihexyphenidyl hydrochloride, 12 mg; clonazepam, 3 mg; and baclofen, 30 mg); however, there was no improvement. Botulinum treatment was also ineffective. During the same period, the right MCA stenosis was observed on head magnetic resonance angiography (MRA). The patient was referred to our hospital and her medical history revealed a persistent depressive disorder. The patient had been in a psychiatric clinic and treated since the age of 22 years. Her current doctor has been treating her since she was 30 years old. Her mother, who had already died, had a delusional disorder. The patient has lived with her mother since childhood. When she was 17 years old, she wanted to go to college because her academic performances were good; however, she dropped out of high school at the request of her mother. Her energy was often exhausted partly because of frequent relocations due to neighborhood troubles caused by her mother, who died when she was 42 years old. She had little interpersonal interaction and often failed when she tried to interact. She frequently made damaging remarks and was often allowed to make detrimental statements about it. She often stayed at home because of her interpersonal relationships and fear of going out. Based on the above-mentioned course and no psychiatric symptoms that could be concluded as delusions, her psychiatric symptoms were judged to be mainly depressed, and she was treated internally as a persistent depressive disorder. She was prescribed propericiazine (15 mg), ramelteon (8 mg), etizolam (2 mg), brotizolam (0.25 mg), mirtazapine (15 mg), nortriptyline hydrochloride (30 mg), levomepromazine hydrochloride (15 mg), and eszopiclone (2 mg) when she visited our hospital. She consumed beer daily (700 mL/day) but had not smoked for 10 years.
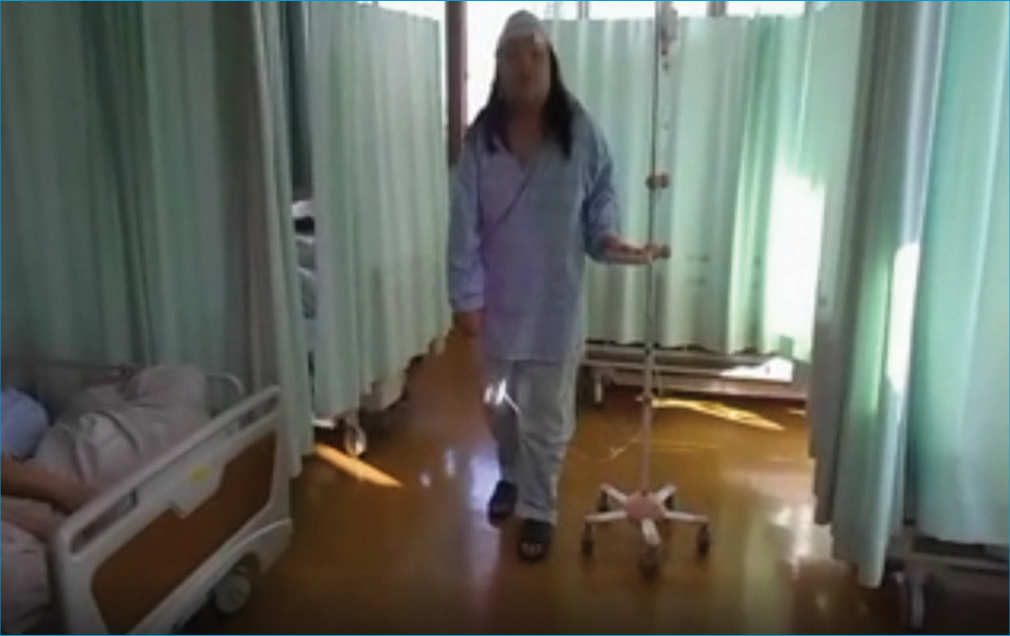
Involuntary neurological movements, including cervical back bending and truncal forward bending, were observed while walking [
Supplementary Video 1
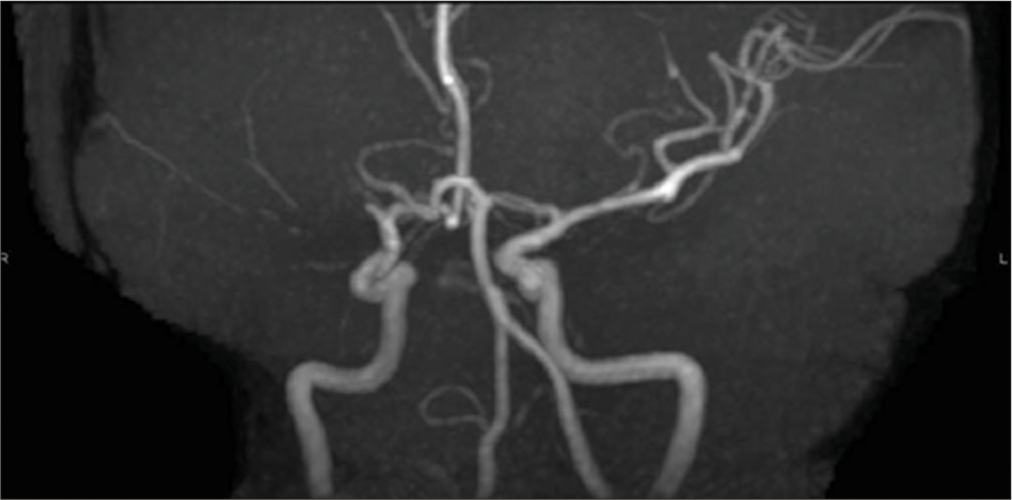
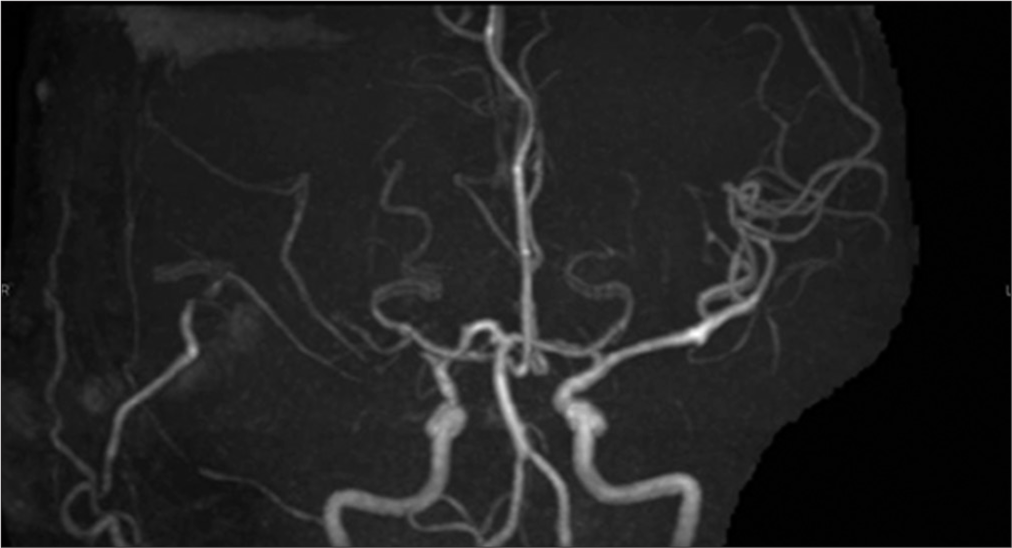
When the patient visited our hospital, MRA revealed the right MCA occlusion [
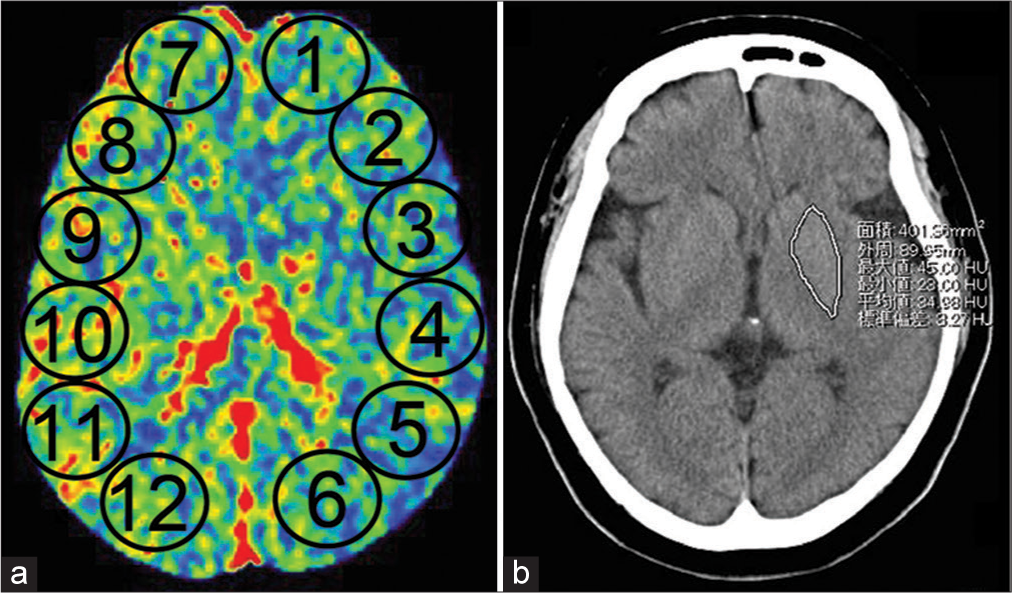
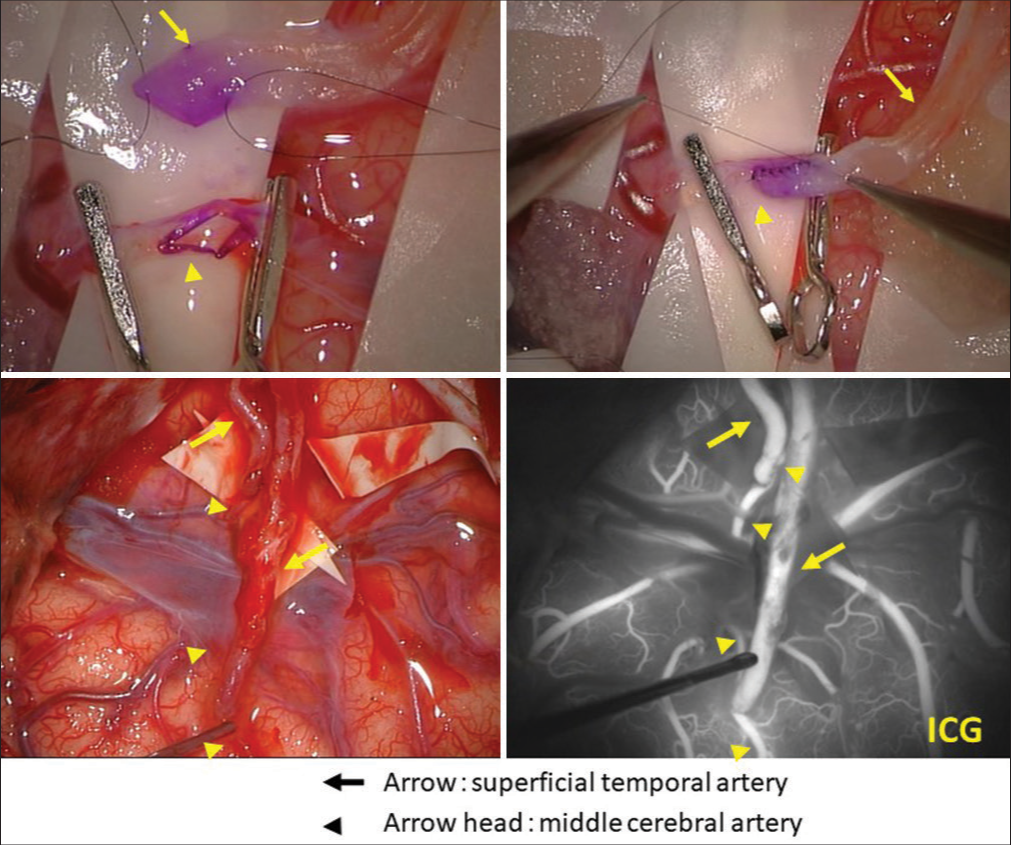
Figure 3:
(a) Region of interest for cerebral blood flow measurement using perfusion computed tomography. Comparison of the average cerebral blood flow in ②, ③, ④, and ⑤ (left MCA territory) with that in ⑧, ⑨, ⑩, and ⑪ (right MCA territory). (b) Region of interest for blood flow measurement in the basal ganglia through perfusion computed tomography. Tracing of the basal ganglia and comparison of the blood flow on the left and right sides.
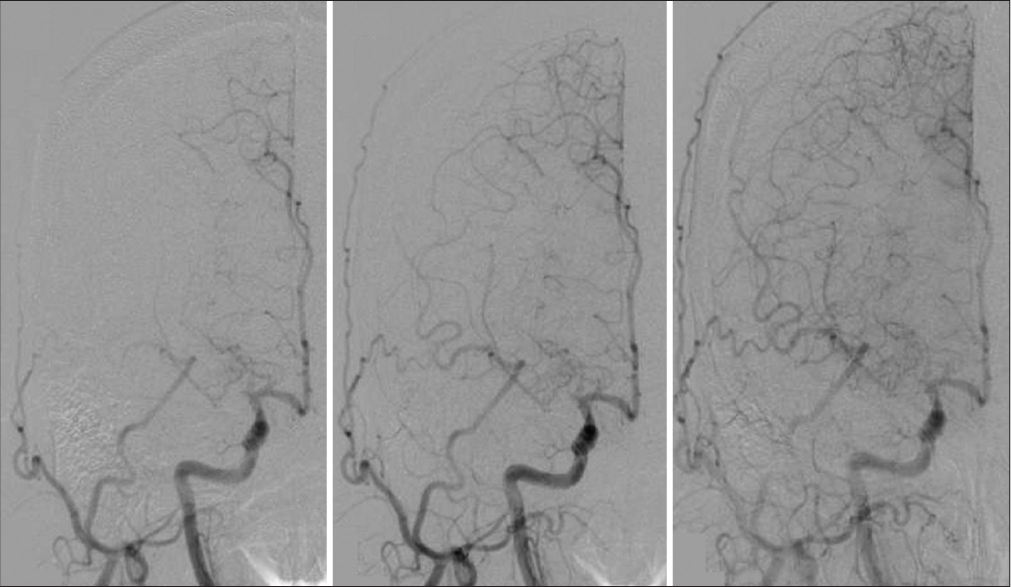
We performed superficial temporal artery (STA)-MCA bypass surgery because we believed that the dystonia was due to right MCA stenosis [
Supplementary Video 2
Supplementary Video 3
DISCUSSION
We encountered a case of truncal dystonia associated with decreased MCA blood flow. Few reports of revascularization for involuntary movements associated with decreased MCA blood flow exist. Chung et al. reported a case of a 26-year-old woman with involuntary movements of the right upper and lower limbs with 48% stenosis in the MCA. In this case, the MCA was completely occluded 1 year later, and the symptoms disappeared after bypass surgery.[
Dystonia may be characterized by two main pathophysiological abnormalities: reduced excitability of inhibitory systems at many levels of the sensorimotor systems and increased plasticity of neural connections in sensorimotor circuits, resulting in overall cortical hyperexcitability.[
Similar conditions have been reported for dystonia, hemiballismus, and limb shaking associated with moyamoya disease.[
The indications for bypass surgery for MCA stenosis during acute cerebral ischemia are controversial. The JET study in Japan has reported that symptomatic cases with a CBF of 80% or less are indicated for surgery.[
We performed revascularization and obtained good clinical outcomes; however, the pathophysiology of dystonia secondary to MCA ischemia is unknown. The pathophysiology of dystonia is being investigated by neurophysiological methods because there are few pathological reports, and neuronal loss is rarely reported.[
Here, we reported a case of truncal dystonia with MCA occlusion that improved with revascularization. On the other hand, this case has a long-term persistent depressive disorder, and the relationship between involuntary movements and persistent depressive disorder cannot be completely ruled out, which is a limitation. Revascularization therapy may be a treatment option for dystonia with ischemia in the MCA region, but further studies may be needed to determine the correct treatment protocol.
CONCLUSION
Revascularization therapy may be a treatment option for dystonia with ischemia in the MCA region.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
SUPPLEMENTARY VIDEOS
References
1. Andrew J, Fowler CJ, Harrison MJG. Stereotaxic thalamotomy in 55 cases of dystonia. Brain. 1983. 106: 981-1000
2. Chung SJ, Lee HS, Yoo HS, Kim KM, Lee KJ, Kim JS. A case of isolated middle cerebral artery stenosis with hemichorea and moyamoya pattern collateralization. J Mov Disord. 2013. 6: 13-6
3. Defebvre L, Krystkowiak P. Movement disorders and stroke. Rev Neurol. 2016. 172: 483-7
4. Dinkelbach L, Hartmann CJ, Mathys C, Wojtecki L, Hänggi D, Südmeyer M. Cervical dystonia caused by focal putaminal ischemia. Ann Clin Transl Neurol. 2015. 2: 1029-31
5. Etgen T, Mühlau M, Gaser C, Sander D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry. 2006. 77: 1017-20
6. Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998. 78: 1-10
7. Gibb WR, Lees AJ, Marsden CD. Pathological report of four patients presenting with cranial dystonias. Mov Disord. 1988. 3: 211-21
8. Greene S, Bansal L, Coffman KA, Nardone R, Zuccoli G. Pial synangiosis ameliorates movement disorders in the absence of prior stroke in moyamoya disease. J Child Neurol. 2016. 31: 646-51
9. Im SH, Oh CW, Kwon OK, Cho BK, Chung YS, Han DH. Involuntary movement induced by cerebral ischemia: Pathogenesis and surgical outcome. J Neurosurg. 2004. 100: 877-82
10. 11. Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: The basal ganglia and the cerebellum?. Neurology. 2006. 67: 1740-41 12. Kamijo K, Matsui T. Dramatic disappearance of moyamoya disease-induced chorea after indirect bypass surgery. Neurol Med Chirurg. 2008. 48: 390-3 13. Kumar S, Sharma S, Jhobta A, Sood RG. Dystonia an unusual presentation in pediatric Moyamoya disease: Imaging findings of a case. J Pediatr Neurosci. 2016. 11: 115-7 14. Matsumoto S, Nishimura M, Shibasaki H, Kaji R. Epidemiology of primary dystonias in Japan comparison with Western countries. Mov Disord. 2003. 18: 1196-8 15. Mehanna R, Jankovic J. Movement disorders in cerebrovascular disease. Lancet Neurol. 2013. 12: 597-608 16. Ondo WG, Desaloms JM, Jankovic J, Grossman RG. Pallidotomy for generalized dystonia. Mov Disord. 1998. 13: 693-8 17. Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009. 132: 2871-7 18. Roman Casul YA, Humbert ML, Farooqui A, Shukla AW, Nagaraja N. Dystonia as a presenting feature of acute ischemic stroke: A case report and literature review. Cureus. 2021. 13: e17272 19. Shibata H, Hayashi Y, Yoshikura N, Yamada M, Kimura A, Shimohata T. Clinical findings of a patient with hemiballism after superficial temporal artery-middle cerebral artery anastomosis for idiopathic middle cerebral artery stenosis. Rinsho Shinkeigaku. 2019. 59: 829-33 20. Sugita Y, Funaki T, Takahashi JC, Takagi Y, Fushimi Y, Kikuchi T. Reversible striatal hypermetabolism in chorea associated with moyamoya disease: A report of two cases. Childs Nerv Syst. 2016. 32: 2243-7 21. Tibussek D, Mayatepek E, Klee D, Koy A. Post stroke hemidystonia in children: A neglected area of research. Mol Cell Pediatr. 2015. 2: 14 22. Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005. 352: 459-67 23. Zheng W, Wanibuchi M, Onda T, Liu H, Koyanagi I, Fujimori K. A case of moyamoya disease presenting with chorea. Childs Nerv Syst. 2006. 22: 274-8 24. Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. 1988. 38: 702-6