- Department of Neurosurgery, “Riuniti” Hospital, Foggia, Italy,
- Department of Neurosurgery, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany,
- Department of Neurosurgery, University of Foggia, Foggia, Italy
- Unit of Medical Oncology and Biomolecular Therapy, University of Foggia, Foggia, Italy,
- Department of Neurosurgery, Baroda Medical College, Vadodara, Gujarat, India.
Correspondence Address:
Nicola Pio Fochi, Department of Neurosurgery, University of Foggia, Foggia, Italy.
DOI:10.25259/SNI_674_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Augusto Leone2, Nicola Pio Fochi3, Veronica Di Napoli3, Guido Giordano4, Matteo Landriscina4, Kashyap Patel5, Francesco Carbone2. Tumor treating fields for the treatment of glioblastoma: Current understanding and future perspectives. 10-Nov-2023;14:394
How to cite this URL: Antonio Colamaria1, Augusto Leone2, Nicola Pio Fochi3, Veronica Di Napoli3, Guido Giordano4, Matteo Landriscina4, Kashyap Patel5, Francesco Carbone2. Tumor treating fields for the treatment of glioblastoma: Current understanding and future perspectives. 10-Nov-2023;14:394. Available from: https://surgicalneurologyint.com/surgicalint-articles/12632/
Abstract
Background: This review focuses on the recently published evidence on tumor treating fields (TTFields) administered alone or in combination with locoregional and systemic options for treating glioblastoma (GBM) in the past ten years. The aim is to critically summarize the novelty and results obtained with this innovative tool, which is becoming part of the armamentarium of neurosurgeons and neuro-oncologists.
Methods: A comprehensive search and analysis were conducted on pivotal studies published in the past ten years. Furthermore, all completed clinical trials, whose results were published on clinicaltrials.gov, were examined and included in the present review, encompassing both recurrent (r) and newly diagnosed (n) GBM. Finally, an additional examination of the ongoing clinical trials was also conducted.
Results: Recent trials have shown promising results both in patients with nGBM and rGBM/progressive (rGBM), leading to Food and Drug Administration approval in selected patients and the Congress of Neurological Surgeons to include TTFields into current guidelines on the management of GBM (P100034/S001-029). Recently, different randomized trials have demonstrated promising results of TTFields in combination with standard treatment of n- and rGBM, especially when considering progression-free and overall survival, maintaining a low rate of mild to moderate adverse events.
Conclusion: Optimal outcomes were obtained in nGBM and progressive disease. A possible future refinement of TTFields could significantly impact the treatment of rGBM and the actual standard of care for GBM, given the better safety profile and survival effects.
Keywords: Brain tumor, Clinical trial, Glioblastoma, High-grade glioma, Tumor treating fields
INTRODUCTION
High-grade gliomas represent the most frequent yet malignant type of brain tumor, with a median overall survival (OS) of only 14.6 months following current standard therapy comprising gross total surgical resection combined with adjuvant radiation therapy (RT) and systemic chemotherapy.[
In the pursuit of achieving longer OS and progression-free survival (PFS) as well as higher objective response rates (ORRs) with limited neurological and systemic impairment, a pivotal role is played by advanced preoperative mapping techniques, allowing the resection of previously inaccessible tumors.[
Notwithstanding, the recent advancements allowed by the vibrant and intense pre- and clinical pharmacological research and the numerous technological advancements geared toward more efficient management of GBM, survival, and local disease control are still unsatisfactory. Since the approval for clinical practice for rGBM and newly diagnosed GBM (nGBM) in 2011 and 2015, respectively,[
PRINCIPLES AND FUNCTIONING OF TTFIELDS
Despite numerous advancements in cancer treatment and the introduction of innovative therapeutic options, the mortality rate for GBM remains dismal.[
Therefore, a similar study by Porat et al.[
In 2016, Kim et al.[
Notwithstanding the remaining uncertainties regarding the exact mechanisms of action of TTFields, an examination of preclinical studies allows us to summarize these antineoplastic effects into the following categories: (1) the interruption of the microtubule formation, achieved by impairing the assembly of a functioning mitotic spindle and a dielectrophoretic effect, with TTFields generating a non-uniform field which forces organelles and polar macromolecules to move in a certain direction, therefore separating them from their daughter cells,[
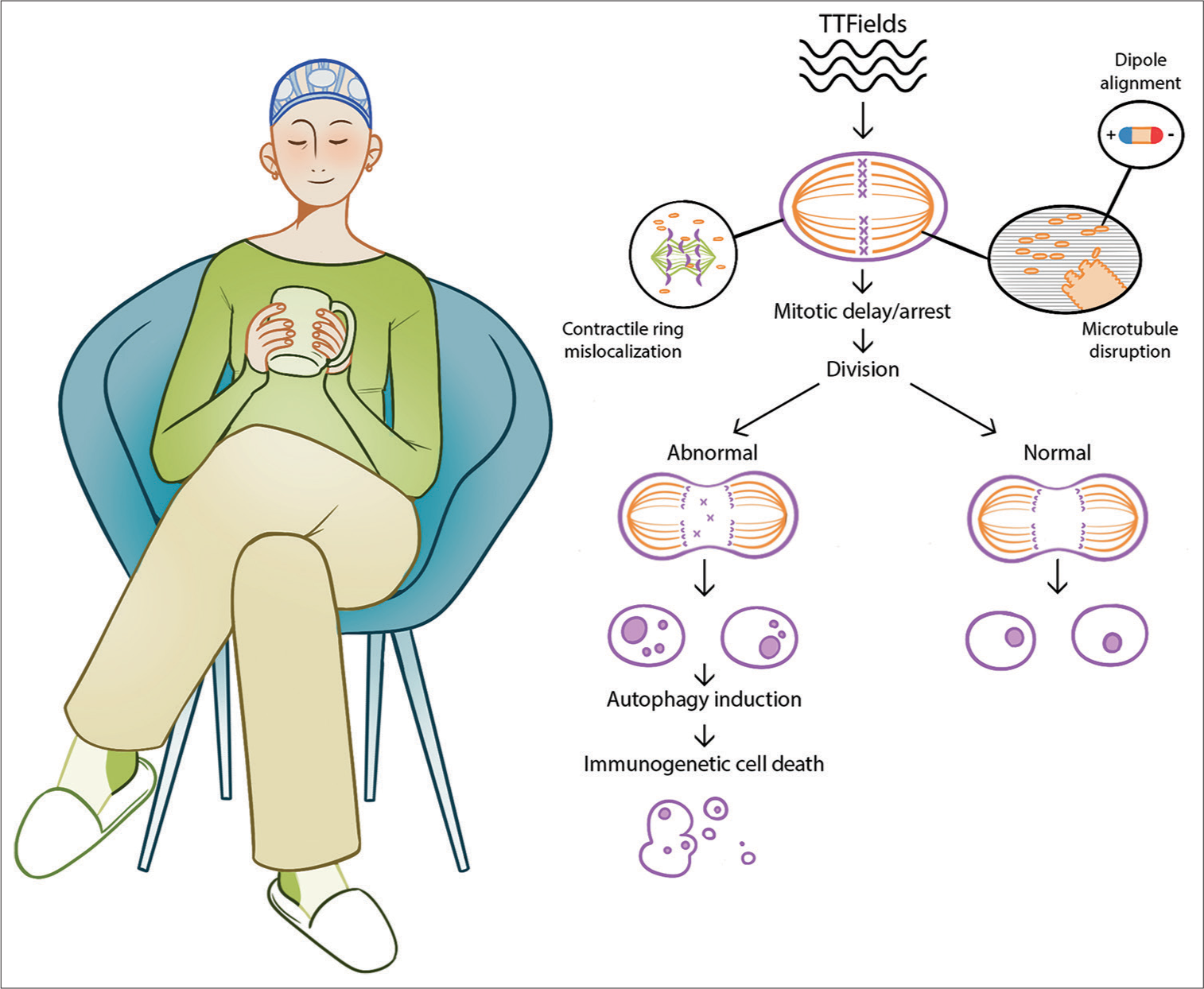
A schematic representation of the functioning mechanisms of TTFields is shown in
Figure 1:
Schematic representation of the functioning mechanisms of tumor treating fields (TTFields). In the left part of the image, a blue mesh can be seen applied on the head of the patient performing her daily activities, without restrictions. This mesh represents the TTFields transducer array which is connected to a battery-operated field-generating device (not shown). Transducer arrays deliver low-intensity intermediate-frequency alternating electric fields and monitor the temperature of the scalp, to avoid heat-induced skin reactions. A schematic description of the physical mechanisms of TTFields is provided on the right side of the image. TTFields exert directional forces and result in abnormal spindle formation and subsequent mitotic arrest or delay, possibly due to improper attachment of chromosomes to the spindle fibers. In dividing cells, this leads to an abnormal arrest of the anaphase during the mitotic cycle, subsequently inducing autophagy and downstream immunogenic cell death.
This innovative therapy showed impressive results when administered alone and demonstrated synergistic effects when coupled with commonly used systemic agents in various solid tumors, including gliomas.[
Moreover, preclinical studies revealed that combining TTFields with a standard-of-care drug could significantly reduce the doses of the latter, allowing the final therapy to be as effective as before, or even more, while significantly decreasing drug-related toxicities.[
First, Giladi et al.[
Moreover, Silginer et al.[
As demonstrated, TTFields have antiproliferative properties both in vivo and in vitro.[
ROLE OF TTFIELDS IN NGBM
Following the results of pre-and clinical trials, the FDA approved TTFields in 2015 as a therapeutic option for nGBM in combination with the standard of care, consisting of total resective surgery, followed by adjuvant chemoradiotherapy and maintenance systemic therapy.[
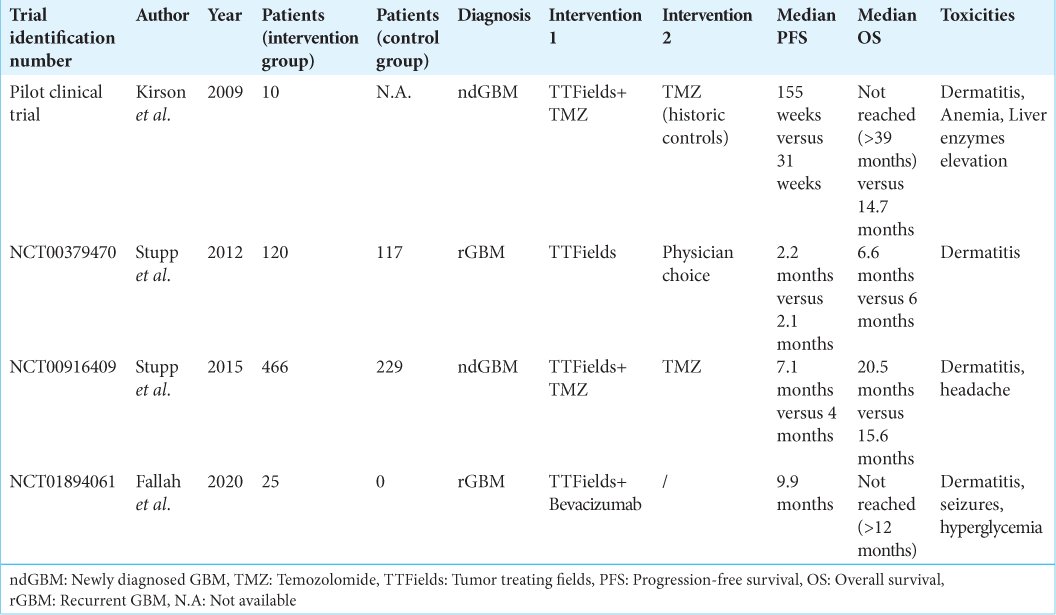
Since then, this novel treatment option has been investigated as a sole intervention and adjuvant management of diffuse high-grade gliomas. For instance, the promising application of TTFields has been documented in several pilot studies and clinical trials.[
Following a further and more extensive trial (EF-11) confirming the overall comparable results in OS and clinical response between TTFields and standard-of-care therapy, the device received FDA approval in 2011 for the treatment of rGBM and in 2015 for nGBM.[
The compliance to the treatment is an additional feature that may interfere with TTFields effect modifying clinical outcomes; in fact, the anti-mitotic effect induced by TTFields may be limited due to insufficient use of the device. The percentage of monthly TTFields compliance collected directly from each device’s internal computerized log file is the primary outcome of a randomized (2:1), open-label trial, the EF-14 phase III.[
Interestingly, modern research is also geared toward including molecular footprints of GBM in the patient selection process, which serves as prognostic as well as therapeutical efficacy predicting factors when administering TTFields. Most commonly investigated molecular markers include methylguanine methyltransferase (MGMT) methylation status, epidermal growth factor receptor amplification, chromosome 1p/19q codeletion, and IDH1 mutation.[
Furthermore, many researchers hold the hypothesis speculating that several subpopulations of cancer stem cells in GBM are responsible for tumor initiation and progression.[
USE OF TTFIELDS IN THE PROGRESSIVE DISEASE
Despite recent advancements in the neuro-oncological field, the prognosis for rGBM remains dismal, hindered by the paucity of effective therapeutic options and the absence of definitive guidelines for its management.[
Consequently, an increasing number of alternative therapeutic approaches are being considered, including bevacizumab, an anti-vascular endothelial growth factor drug; alkylating agents such as carmustine or nitrosoureas; and procarbazine or TTFields.[
A randomized phase III trial (EF11 trial) evaluated the efficacy of TTFields in rGBM compared to the actual standard of care.[
Following the efficacy of this innovative tool in the abovementioned trial, a Patient Registry Dataset (PRiDe) was carried out between October 2011 and November 2012 to evaluate the efficacy and safety of TTFields for rGBM in 457 adult patients, whose results were then compared to the EF-11 trial.[
To further investigate the efficacy of this novel therapy, a case series of patients with rGBM treated with TTFields was published in 2012 by Rulseh et al. [
Another clinical trial involving 25 patients was conducted between 2013 and 2017, examining a combination of bevacizumab and TTFields for progressive disease.[
The enrolled prospective clinical studies are summarized in
ONGOING CLINICAL TRIALS
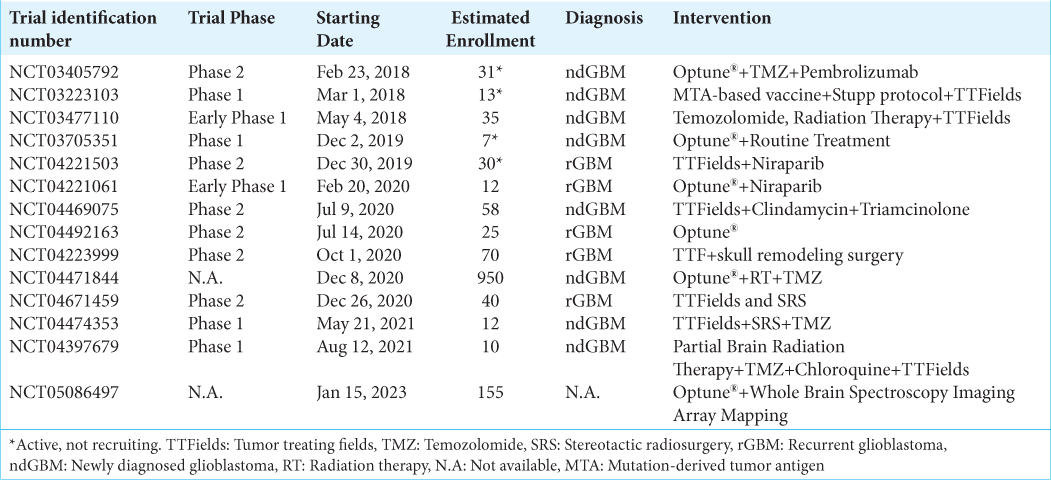
In addition to the experiences that have led to the FDA approval of TTFields for rGBM and newly diagnosed disease, the following section explores ongoing clinical trials to highlight the new frontiers and future perspectives of this technology for treating high-grade brain tumors [
A phase II clinical trial, namely, TaRRGET (NCT04671459), has already enrolled 40 patients and aims to concomitantly administer SRS and TTFields, not only to minimize toxicities but also to increase tumor sensitivity to radiation, a consequence of the TTFields action in disrupting DNA repair and enhancing immunogenic cell death. For that purpose, SRS will be delivered seven days after starting TTFields and with a 5-day regimen, during which the TTFields will be interrupted and restarted immediately after. To accurately analyze the efficacy of this combination therapy, fluoroethyltyrosine positron emission tomography imaging will be used to define tumor volume at recurrence. While 1-year survival rate remains the primary outcome, it is noteworthy to mention that secondary outcomes also include radiation necrosis range, steroid needs until treatment failure, and failure patterns in an effort to assess possible connections between the location of failure and target volume.
An open-label and single-arm pilot study (NCT04492163) is being conducted on 25 patients with an innovative medical device named Optune, which has already received FDA approval as well as a CE mark for the treatment of progressive and nGBM. Requirements include using the device for a minimum of 18 h a day, allowing the remaining time for hygiene and other personal necessities. The traditional version of the device consists of 4 high-intensity transducer arrays delivering 200kHz TTFields to the brain. This clinical trial aims to test new-generation arrays that reduce skin heating, enabling a larger delivery of high-intense TTFields. This pilot study’s purpose is to assess whether the maintenance of high-intensity frequencies, guaranteed by the decreased skin side effects of the new device, improves the final clinical outcome of the patients enrolled. Unlike the previous clinical trial, the present focuses on examining the clinical therapeutic effects of TTFields alone, therefore, in the absence of any other therapeutic procedure, including chemotherapy and radiotherapy.
Another ongoing, phase 2/3 Danish study (NCT04223999) is actively recruiting patients with rGBM who will be randomized 1:1 to receive either skull remodeling-surgery, TTFields and best practice oncological therapy, or TTFields and best oncological therapy alone (control arm). The purpose is to test this minor and safe surgical procedure, creating small burr holes in the patient’s skull over the tumor location, combined with standard TTFields at first progression. The background of this investigation is based on the local resistance caused by the skull, which would be sensibly reduced by funneling the electricity through the path of least resistance, namely, the adequately placed burr holes, approximately 15 mm in diameter. Primary outcome measures will be OS at 12 months, while median OS, PFS, and PFS at six months are expected to be calculated with an estimated follow-up time of 18 months and a total study duration of 36 months.
A pivotal, randomized, and open-label study (NCT04471844) is still in a state of recruitment with an estimated enrolled population of 950 patients until August 2026. The goal of the trial is to test the effectiveness and safety of Optune with a concomitant RT and TMZ in nGBM patients and compared it to the control arm that underwent radiochemotherapy (randomized 1:1). In both arms, TMZ concomitant with TTFields at 200 kHz to the brain is applied as maintenance therapy. OS is the primary outcome of the study, while secondary outcomes include PFS (at 6 and 12 months and up to 5 ys) and next PFS, calculated in case of second tumor progression and associated with the evaluation of pathological changes in resected GBM tumors during the study treatment. Further, secondary points are 1- and 2-year survival rates, Objective response rate (ORR) based on the Response Assessment in Neuro-Oncology (RANO) criteria, the quality of life tested with the EORTC QLQ C-30 questionnaire, the NANO scale for neurological assessment, and adverse events expressed in terms of frequency and severity. In addition, one of the aims of the trial is to understand whether the TTFields dose delivered to the tumor correlates with the OS. The dependency will be examined in both groups.
In a non-randomized trial (NCT03405792), close to being completed (February 2023) with 31 patients enrolled, pembrolizumab is combined with TMZ plus Optune; the purpose of the study is to determine whether the triple combination succeeds in increasing PFS in patients with nGBM. Four to six weeks after completing standard treatment of GBM, monthly cycles (from 6 to 12 cycles) of adjuvant TMZ are administered with concomitant application of TTFields. After cycle 2 of adjuvant TMZ and Optune, in the first arm, pembrolizumab is added to be administered every three weeks for two years in case of no progression of disease or poor adverse events. The primary outcome is PFS, investigated in the triple combination arm and historical arm to compare the results and clarify the potential additional effects that pembrolizumab may add to the combined therapy. OS, augmentation of TTFields-initiated glioma-specific immune reaction by pembrolizumab, and toxicity and tolerability examined by the Common Terminology Criteria for Adverse Events version 4.0 are the secondary outcomes measured. All estimated outcomes are assessed up to 24 months except for OS, which is extended up to 5 years.
Finally, a non-randomized, phase 2 study actively enrolled patients with rGBM to evaluate the efficacy and safety of neratinib in combination with TTFields. Thirty patients are expected to be enrolled between December 2019 and December 2025 and will be divided into two cohorts depending on the clinical indication for surgical resection. In each cohort, neratinib and TTFields will be administered in combination. The rationale is based on pharmacodynamic pathways: neratinib is a selective inhibitor of the poly-ADP ribose polymerase, a mechanism allowing cells to repair single-strand DNA breaks, whereas TTFields have been demonstrated to induce a downregulation of the BRCA1 signaling and reduce DNA double-strand break repair capacity. A synergistic effect is, therefore, to be expected. The primary outcome of this study is disease control, defined as the achievement of either complete, partial response, or stable disease as defined by modified RANO criteria. Secondary outcomes include number of adverse events, duration of disease control, and objective radiographic response.
In conclusion, the presence of several ongoing clinical trials, broadly different from one another, suggests the applicability of TTFields in various management protocols for progressive and nGBM, further demonstrating the importance of this innovative device in promising optimal results while maintaining a sustainable safety profile.
SAFETY AND TOXICITIES
When compared to chemotherapy, TTFields showed a significantly lower rate of hematological, gastrointestinal, and infectious adverse events.[
Confirming the previous statement, a recent phase III study[
LIMITATIONS OF THE DEVICE
Although TTFields devices’ portability and non-invasiveness, several limitations prevent this novel tool’s broad acceptance and use. As highlighted by Turner et al.[
Notwithstanding the impact of this novel therapy on GBM treatment, some evaluations have been made and should, therefore, be reported: (1) an absence of a specific biomarker to identify the molecular subgroup most likely to benefit more from this therapy;[
CONCLUSION
Practical advantages of TTFields compared to the other specific oncological treatment include: (i) non-invasiveness of the device and relatively free scheduling of the “device-on period,” allowing the patient to organize his/her routine; (ii) safe addition to the cytotoxic chemotherapy; (iii) absence of any significant negative interaction with radiotherapy whose effects are ultimately enforced by concomitant TTFields administration which delays DNA damage repair;[
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to thank Susanna Damato who created the figure presented in this manuscript, allowing for a clear yet synthetic iconographic representation of the daily application as well as functioning principles of TTFields.
References
1. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015. 129: 829-48
2. Ballo MT, Urman N, Lavy-Shahaf G, Grewal J, Bomzon Z, Toms S. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: A large-scale numerical simulation-based analysis of data from the phase 3 EF-14 randomized trial. Int J Radiat Oncol Biol Phys. 2019. 104: 1106-13
3. Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006. 444: 756-60
4. Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016. 18: 1129-36
5. Carrieri FA, Smack C, Siddiqui I, Kleinberg LR, Tran PT. Tumor treating fields: At the crossroads between physics and biology for cancer treatment. Front Oncol. 2020. 10: 575992
6. Ceresoli GL, Aerts JG, Dziadziuszko R, Ramlau R, Cedres S, van Meerbeeck JP. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019. 20: 1702-9
7. Chen C, Xu H, Song K, Zhang Y, Zhang J, Wang Y. Tumor treating fields combine with temozolomide for newly diagnosed glioblastoma: A retrospective analysis of Chinese patients in a single center. J Clin Med. 2022. 11: 5855
8. Clark PA, Gaal JT, Strebe JK, Pasch CA, Deming DA, Kuo JS. The effects of tumor treating fields and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. J Clin Neurosci. 2017. 36: 120-4
9. Davies AM, Weinberg U, Palti Y. Tumor treating fields: A new frontier in cancer therapy. Ann NY Acad Sci. 2013. 1291: 86-95
10. Davis ME. Glioblastoma: Overview of disease and treatment. Clin J Oncol Nurs. 2016. 20: S2-8
11. Fabian D, Guillermo Prieto Eibl MD, Alnahhas I, Sebastian N, Giglio P, Puduvalli V. Treatment of glioblastoma (GBM) with the addition of tumor-treating fields (TTF): A Review. Cancers (Basel). 2019. 11: 174
12. Fallah J, Chaudhary RT, Rogers LR, Wei W, Brewer CJ, Peereboom DM. Clinical outcomes of the combination of bevacizumab and TTFields in patients with recurrent glioblastoma: Results of a phase II clinical trial. J Clin Oncol. 2020. 38: 2537-7
13. Giladi M, Munster M, Schneiderman RS, Voloshin T, Porat Y, Blat R. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017. 12: 206
14. Guzauskas GF, Pollom EL, Stieber VW, Wang BC, Garrison LP. Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: A cost-effectiveness study. J Med Econ. 2019. 22: 1006-13
15. Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M. Correlation of O 6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008. 26: 4189-99
16. Kim EH, Kim YH, Song HS, Jeong YK, Lee JY, Sung J. Biological effect of an alternating electric field on cell proliferation and synergistic anti-mitotic effect in combination with ionizing radiation. Oncotarget. 2016. 7: 62267-79
17. Kirson ED, Dbalý V, Tovarys F, Vymazal J, Soustiel JF, Itzhaki A. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007. 104: 10152-7
18. Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004. 64: 3288-95
19. Kirson ED, Schneiderman RS, Dbalý V, Tovarys F, Vymazal J, Itzhaki A. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009. 9: 1
20. Krigers A, Pinggera D, Demetz M, Kornberger LM, Kerschbaumer J, Thomé C. The routine application of tumor-treating fields in the treatment of glioblastoma WHO IV. Front Neurol. 2022. 13: 900377
21. Lacouture ME, Davis ME, Elzinga G, Butowski N, Tran D, Villano JL. Characterization and management of dermatologic adverse events with the NovoTTF-100A system, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014. 41: S1-14
22. Lassman AB, Joanta-Gomez AE, Pan PC, Wick W. Current usage of tumor treating fields for glioblastoma. Neurooncol Adv. 2020. 2: vdaa069
23. Lehrer EJ, Ruiz-Garcia H, Nehlsen AD, Sindhu KK, Estrada RS, Borst GR. Preoperative stereotactic radiosurgery for glioblastoma. Biology (Basel). 2022. 11: 194
24. Leone A, Colamaria A, Fochi NP, Sacco M, Landriscina M, Parbonetti G. Recurrent glioblastoma treatment: State of the art and future perspectives in the precision medicine era. Biomedicines. 2022. 10: 1927
25. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
26. Lukas RV, Ratermann KL, Wong ET, Villano JL. Skin toxicities associated with tumor treating fields: Case based review. J Neurooncol. 2017. 135: 593-9
27. McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ. 2021. 374: n1560
28. Mehta M, Wen P, Nishikawa R, Reardon D, Peters K. Critical review of the addition of tumor treating fields (TTFields) to the existing standard of care for newly diagnosed glioblastoma patients. Crit Rev Oncol Hematol. 2017. 111: 60-5
29. Mirza FA, Shamim MS. Tumour treating fields (TTFs) for recurrent and newly diagnosed glioblastoma multiforme. J Pak Med Assoc. 2018. 68: 1543-5
30. Mittal S, Klinger NV, Michelhaugh SK, Barger GR, Pannullo SC, Juhász C. Alternating electric tumor treating fields for treatment of glioblastoma: Rationale, preclinical, and clinical studies. J Neurosurg. 2018. 128: 414-21
31. Moser JC, Salvador E, Deniz K, Swanson K, Tuszynski J, Carlson KW. The mechanisms of action of tumor treating fields. Cancer Res. 2022. 82: 3650-8
32. Mumblat H, Martinez-Conde A, Braten O, Munster M, Dor-On E, Schneiderman RS. Tumor Treating Fields (TTFields) downregulate the Fanconi Anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer. 2021. 160: 99-110
33. Omar AI. Tumor treating field therapy in combination with bevacizumab for the treatment of recurrent glioblastoma. J Vis Exp. 2014. 92: e51638
34. Ornelas AS, Porter AB, Sharma A, Knox MG, Marks LA, Wingerchuk DM. What is the role of tumor-treating fields in newly diagnosed glioblastoma?. Neurologist. 2019. 24: 71-3
35. Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: Increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015. 38: E3
36. Pointer KB, Clark PA, Zorniak M, Alrfaei BM, Kuo JS. Glioblastoma cancer stem cells: Biomarker and therapeutic advances. Neurochem Int. 2014. 71: 1-7
37. Porat Y, Giladi M, Schneiderman RS, Blat R, Shteingauz A, Zeevi E. Determining the optimal inhibitory frequency for cancerous cells using tumor treating fields (TTFields). J Vis Exp. 2017. 123: 55820
38. Reardon DA, Desjardins A, Peters K, Gururangan S, Sampson J, Rich JN. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011. 103: 371-9
39. Rulseh AM, Keller J, Klener J, Sroubek J, Dbalý V, Syrůček M. Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World J Surg Oncol. 2012. 10: 220
40. Schneiderman RS, Shmueli E, Kirson ED, Palti Y. TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010. 10: 229
41. Shteingauz A, Porat Y, Voloshin T, Schneiderman RS, Munster M, Zeevi E. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018. 9: 1074
42. Silginer M, Weller M, Stupp R, Roth P. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017. 8: e2753
43. Singh N, Miner A, Hennis L, Mittal S. Mechanisms of temozolomide resistance in glioblastoma-a comprehensive review. Cancer Drug Resist. 2021. 4: 17-43
44. Straube C, Oechsner M, Kampfer S, Scharl S, Schmidt-Graf F, Wilkens JJ. Dosimetric impact of tumor treating field (TTField) transducer arrays onto treatment plans for glioblastomas-a planning study. Radiat Oncol. 2018. 13: 31
45. Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA. 2015. 314: 2535-43
46. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. 2017. 318: 2306-16
47. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012. 48: 2192-202
48. Taphoorn MJ, Dirven L, Kanner AA, Lavy-Shahaf G, Weinberg U, Taillibert S. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018. 4: 495-504
49. Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019. 141: 467-73
50. Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann Oncol. 2017. 28: 1457-72
51. Turner SG, Gergel T, Wu H, Lacroix M, Toms SA. The effect of field strength on glioblastoma multiforme response in patients treated with the NovoTTFTM-100A system. World J Surg Onc. 2014. 12: 162
52. Tuszynski JA, Wenger C, Friesen DE, Preto J. An overview of sub-cellular mechanisms involved in the action of TTFields. Int J Environ Res Public Health. 2016. 13: 1128
53. Voloshin T, Kaynan N, Davidi S, Porat Y, Shteingauz A, Schneiderman RS. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020. 69: 1191-204
54. Zhu P, Zhu JJ. Tumor treating fields: A novel and effective therapy for glioblastoma: Mechanism, efficacy, safety and future perspectives. Chin Clin Oncol. 2017. 6: 41
55. Zorniak M, Clark PA, Leeper HE, Tipping MD, Francis DM, Kozak KR. Differential expression of 2’,3’-cyclic-nucleotide 3’-phosphodiesterase and neural lineage markers correlate with glioblastoma xenograft infiltration and patient survival. Clin Cancer Res. 2012. 18: 3628-36
56. Zhu JJ, Demireva P, Kanner AA, Pannullo S, Mehdorn M, Avgeropoulos N. Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J Neurooncol. 2017. 135: 545-52