- Department of Neurosurgery, Westchester Medical Center, Valhalla, New York,
- Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania,
- Department of Radiation Oncology, University of California San Francisco, San Francisco, California,
- Department of Neurosurgery, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania.
Correspondence Address:
Jared M. Pisapia, Department of Neurosurgery, Westchester Medical Center, Valhalla, New York, United States.
DOI:10.25259/SNI_163_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jared M. Pisapia1, Giscard Adeclat2, Sanford Roberts2, Yun R. Li3, Zarina Ali2, Gregory G. Heuer4, Eric L. Zager2. Tumors of the brachial plexus region: A 15-year experience with emphasis on motor and pain outcomes and literature review. 05-May-2023;14:162
How to cite this URL: Jared M. Pisapia1, Giscard Adeclat2, Sanford Roberts2, Yun R. Li3, Zarina Ali2, Gregory G. Heuer4, Eric L. Zager2. Tumors of the brachial plexus region: A 15-year experience with emphasis on motor and pain outcomes and literature review. 05-May-2023;14:162. Available from: https://surgicalneurologyint.com/surgicalint-articles/12315/
Abstract
Background: Brachial plexus region tumors are rare. In this study, we reviewed our experience with resection of tumors involving or adjacent to the brachial plexus to identify patterns in presentation and outcome.
Methods: We report a retrospective case series of brachial plexus tumors operated on by a single surgeon at a single institution over 15 years. Outcome data were recorded from the most recent follow-up office visit. Findings were compared to a prior internal series and comparable series in the literature.
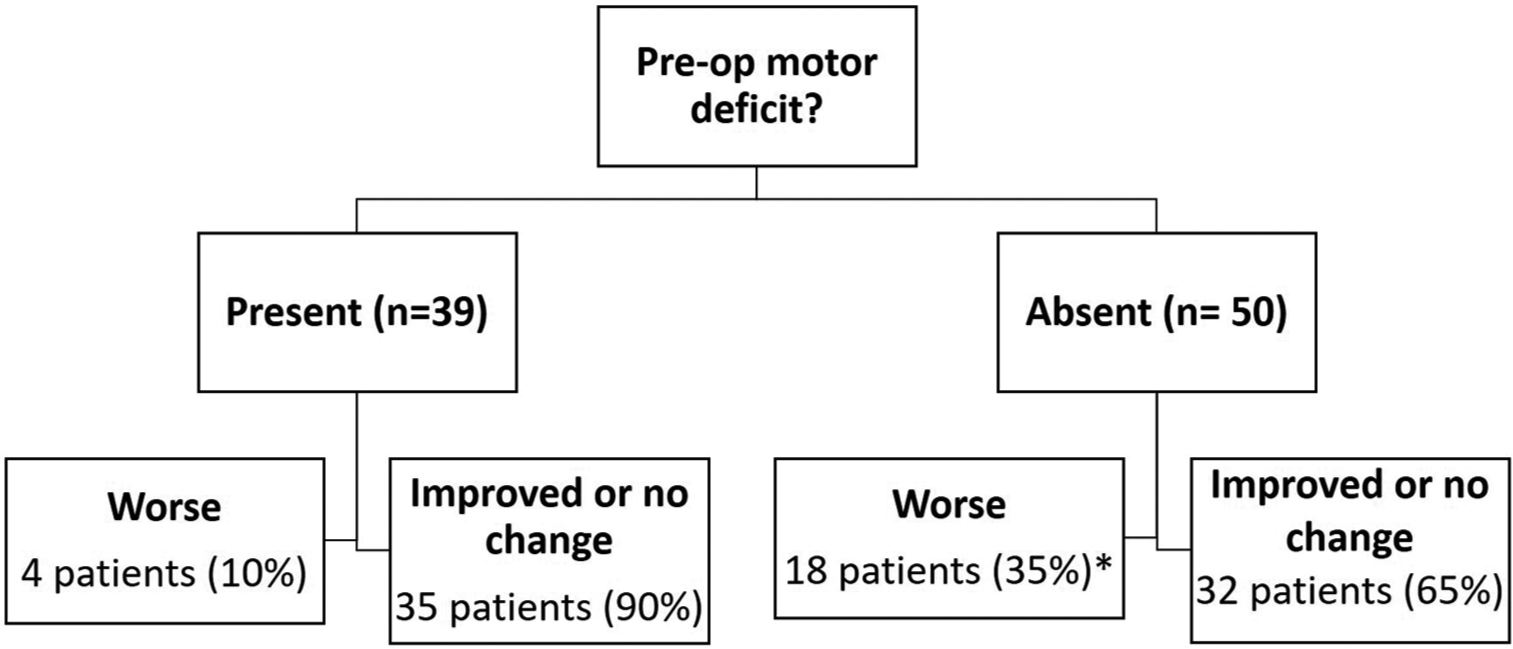
Results: From 2001 to 2016, 103 consecutive brachial plexus tumors in 98 patients met inclusion criteria. Ninety percent of patients presented with a palpable mass, and 81% had deficits in sensation, motor function, or both. Mean follow-up time was 10 months. Serious complications were infrequent. For patients with a preoperative motor deficit, the rate of postoperative motor decline was 10%. For patients without a preoperative motor deficit, the rate of postoperative motor decline was 35%, which decreased to 27% at 6 months. There were no differences in motor outcome based on extent of resection, tumor pathology, or age.
Conclusion: We present one of the largest recent series of tumors of the brachial plexus region. Although the rate of worsened postoperative motor function was higher in those without preoperative weakness, the motor deficit improves over time and is no worse than antigravity strength in the majority of cases. Our findings help guide patient counseling in regard to postoperative motor function.
Keywords: Brachial plexus, Nerve sheath tumor, Neurofibroma, Outcome, Schwannoma, Tumor
INTRODUCTION
Tumors of the brachial plexus region are uncommon, making up no more than 5% of all tumors of the upper extremity and hand.[
Due to the relative rarity of these tumors, we set out to further characterize patterns ranging from clinical presentation to postoperative findings to improve the care of patients with these lesions and to provide prognostic information. Most of the larger series of brachial plexus tumors are from several decades ago,[
MATERIALS AND METHODS
Study design
In this retrospective case series, we reviewed the medical records of patients undergoing surgery for tumors of the brachial plexus region by a single senior neurosurgeon at an academic institution. Subjects were identified from the surgeon’s operative case log. Patients of all ages were included, although the surgeon’s practice is primarily adult-based. In addition to tumors originating from nerves and their coverings, tumors or metastatic lesions of the surrounding region that impinged on the brachial plexus were included in the study. The tumor itself was considered the unit for analysis. Thus, patients with tumors in more than one location within the brachial plexus that was operated on at different time points were included separately in the analysis. Tumors previously resected at outside hospitals were excluded from the analysis. Patients studied in a prior institutional study of brachial plexus tumors were excluded from the current investigation.[
Data extraction
Office notes, radiological images, and pathology reports were reviewed. Data extracted from the medical record included demographics and presenting symptoms, including pain, weakness, and sensory change. Data on surgical approach were collected. In general, an anterior supraclavicular approach was taken for tumors involving the spinal nerves and trunks, whereas an anterior infraclavicular approach was taken for tumors involving the cords and distal plexus elements. Combined approaches and axillary approaches were used in select cases. Neuromonitoring was used intraoperatively. Final tumor pathology was recorded in all cases. For postoperative data, patients were required to have a minimum of 1-month follow-up, which typically was in the form of a formal postoperative visit. Separate analyses were performed for patients with more than 6 months of follow-up. Data from the time of most recent follow-up were collected and included changes in preoperative symptoms, motor strength, and sensation. Pain was numerically rated pre and postoperatively on a visual analog scale ranging from 0 (no pain) to 10 (worst pain possible). Postoperative pain at most recent follow-up was categorized as worse, improved, or unchanged based on the difference between pre- and postoperative pain ratings or lack thereof. Improved or worsened motor strength was considered as a one-point change in manual muscle testing based on the Medical Research Council (MRC) Scale for muscle strength for tested muscles. Gross total resection (GTR) was defined as resection of 95% or more of the mass as determined by the radiology report. Recurrences were defined as patients with tumor growth on postoperative MRI such that additional surgery or treatment was performed.
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics. Subgroup analysis was performed to determine the relationship between motor outcome versus follow-up time (1 month vs. greater than months), pathology (benign vs. malignant/non-neural nerve sheath tumors), extent of resection (subtotal vs. gross total), and age (greater than or less than the median age of all subjects). Chi-square was used to compare categorical variables, and Fisher’s exact test was used for sample sizes of five or fewer within a category. Univariate and multivariate regression were performed to assess the relationship between several variables and improved postoperative motor outcomes. The threshold for statistical significance was set at P < 0.05. Statistical tests were performed using Stata 11 software (College Station, Texas).
RESULTS
Patient and tumor characteristics
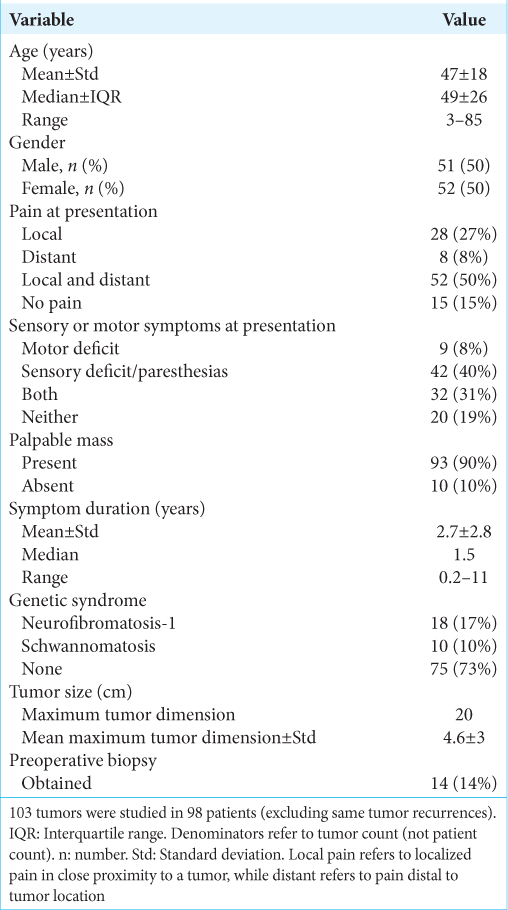
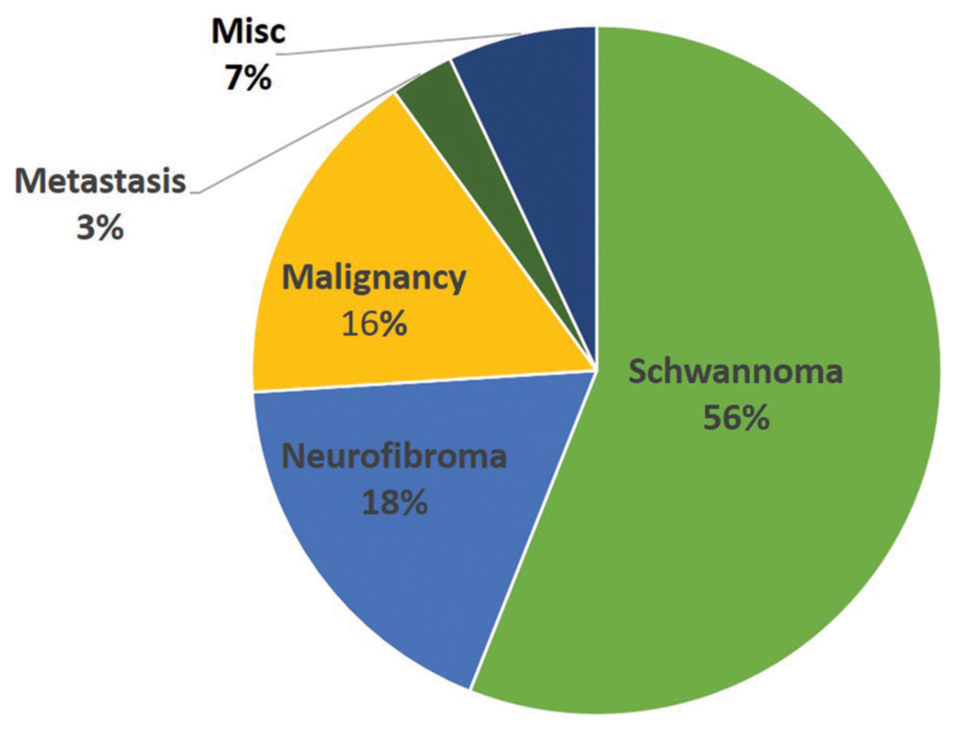
From 2001 to 2016, 120 patients with brachial plexus tumors were identified that underwent surgery by the senior neurosurgeon at a single institution. Among these patients, 22 were excluded due to incomplete or unavailable medical records, leaving 98 patients for analysis. Four patients had more than one surgery due to the presence of two or more separate brachial plexus tumors. Three had NF1 (each with two tumors) and one had schwannomatosis (with three tumors). Thus, 98 patients with 103 tumors were included in the analysis. Other patients had additional tumors, but the additional tumors were operated on at different hospitals and therefore were not included in the analysis. Patient demographics and presenting symptoms are detailed in
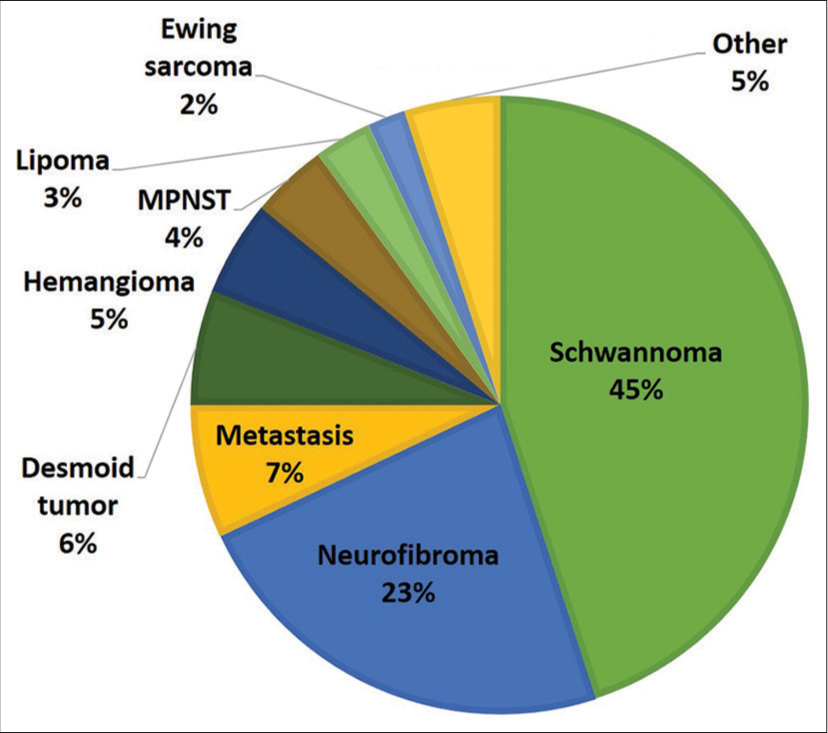
Intraoperative and pathological findings
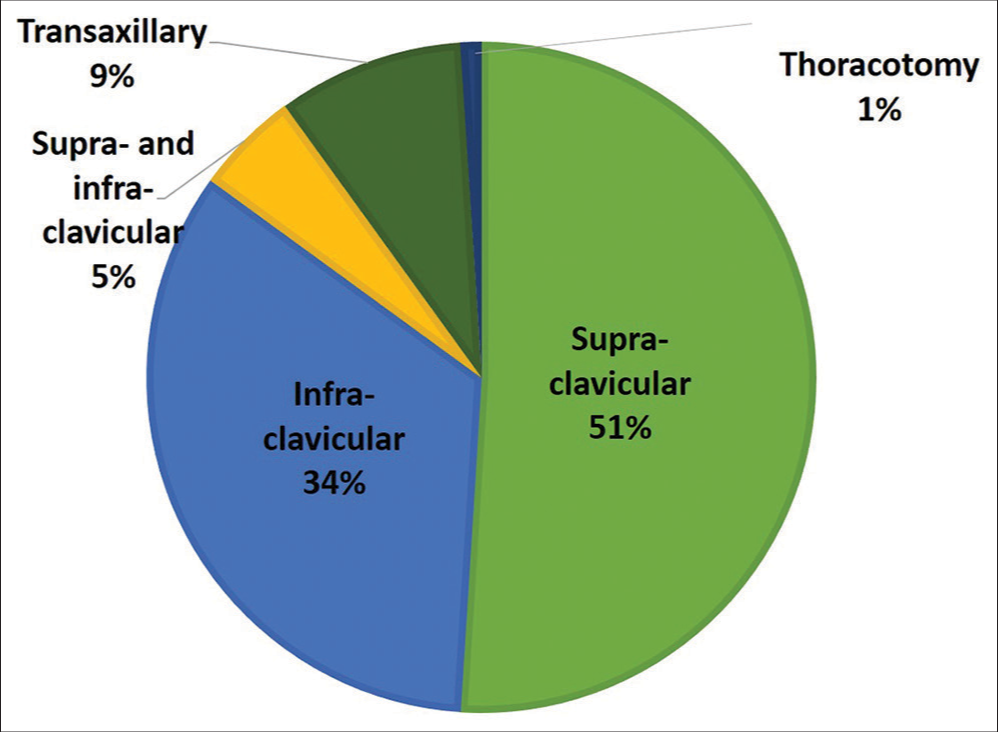
The most common surgical approach was supraclavicular [
Outcome data
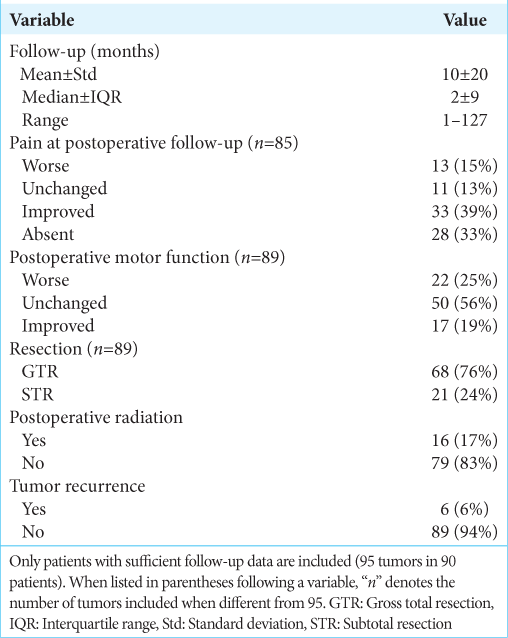
Data regarding outcome variables are listed in
For tumors with follow-up information, 16 patients underwent postoperative radiation for MPNST (n = 5), desmoid tumor (n = 5), metastasis (n = 2), lymphoma (n = 1), atypical teratoid rhabdoid tumor (n = 1), Ewing sarcoma (n = 1), and osteogenic sarcoma (n = 1). Fourteen patients within the most recent follow-up period had postoperative imaging consistent with tumor recurrence, and pathologies included NF (n = 8), schwannoma (n = 3), chordoma (n = 1), desmoid tumor (n = 1), and MPNST (n = 1). Six of these patients required additional treatment, such as repeat surgery or radiation therapy and the remaining patients did not undergo adjuvant therapy at time of most recent follow-up. Surgical complications included phrenic nerve paralysis (n = 1), vessel damage (n = 1) not requiring additional surgical intervention, and hematoma requiring return to the operating room for evacuation (n = 1).
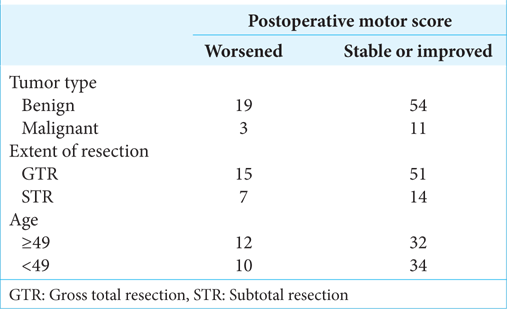
In a subgroup analysis, there was no difference in rates of postoperative motor decline among patients with benign or malignant tumors (Fisher exact statistic = 1) [
Representative cases
Case 1
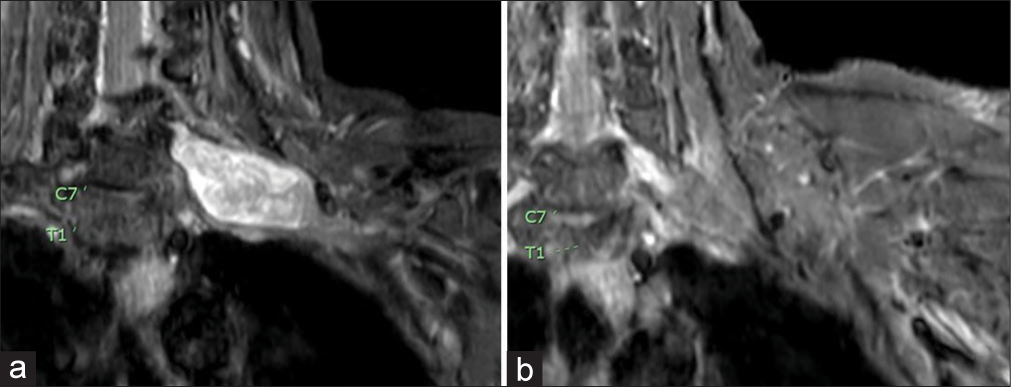
A 60-year-old female presented with an enlarging lump over the left clavicle associated with progressively worsening pain radiating to the back of the left hand. On examination, a Tinel’s sign was present over a palpable supraclavicular mass. MRI of the brachial plexus with contrast showed a 5-cm enhancing mass within the left brachial plexus [
Case 2
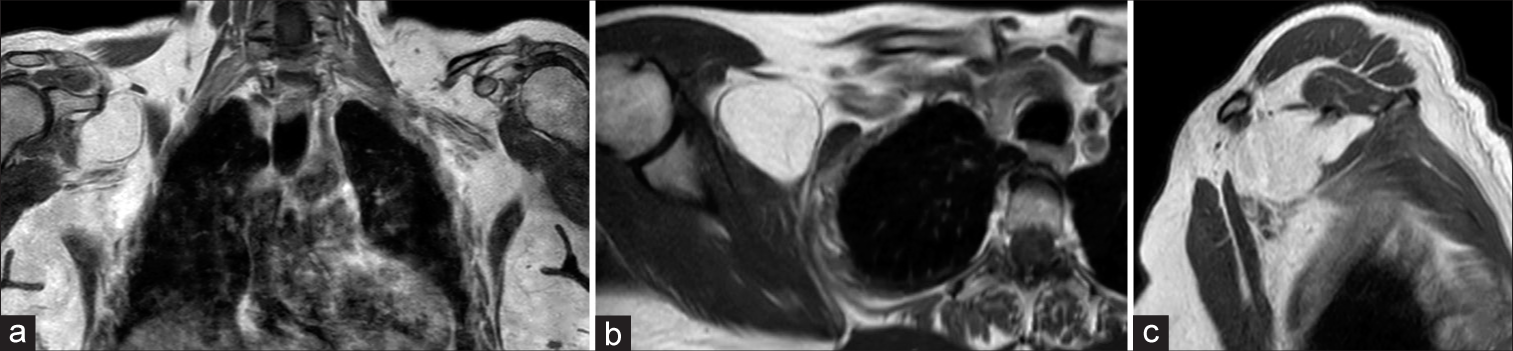
A 70-year-old female presented with progressively worsening right upper extremity paresthesias and a severe band-like sensation around the right thumb. She had normal sensory and motor function on examination. MRI of the brachial plexus showed a 6-cm lesion compressing the lateral cord of the brachial plexus [
DISCUSSION
We present one of the largest recent series of brachial plexus region tumors by a single surgeon at a single academic institution. Our report is unique in that it is an extension of a prior study[
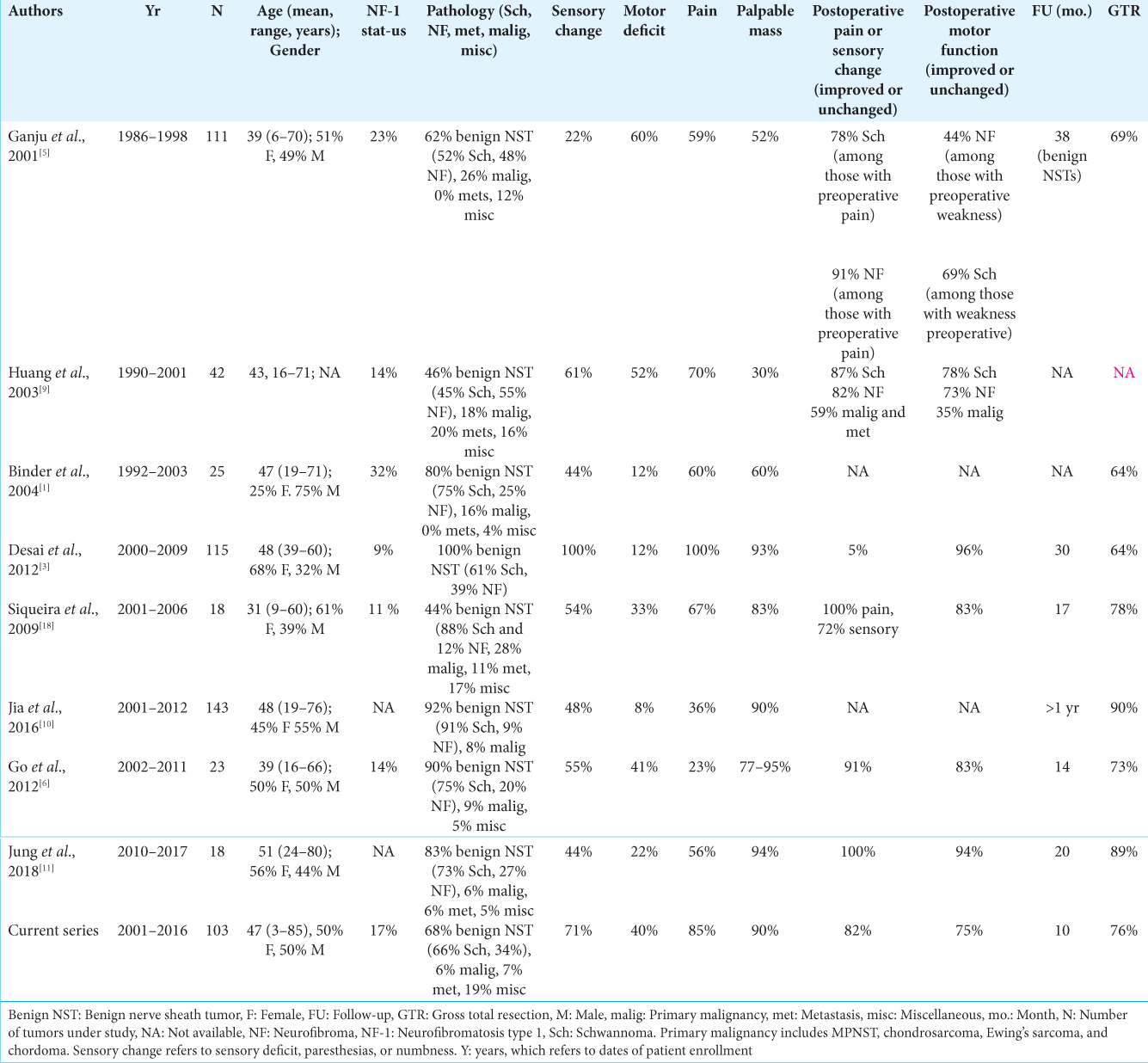
Overall, the distribution of preoperative patient and tumor characteristics in our study is similar to other case series. Most patients undergoing surgery were middle aged, and there was no clear gender predilection. A palpable mass was the most common presenting symptom, which occurred in 90% or more of patients in selected series.[
A special emphasis was placed on motor and pain outcomes in this case series. For patients with a pre-existing preoperative motor deficit, the rate of the motor deficit worsening postoperatively was low (10%). The regression results showing that the presence of a preoperative motor deficit was associated with improved postoperative motor function suggest that motor deficits, possibly related to neural compression, are likely to improve after surgery for tumor removal. For those without a pre-existing motor deficit, the chance of motor function worsening was higher (35%). This rate of decline is likely attributed to the conservative definition of weakness used for the study, which was a one-point decrease in MRC scale for muscle strength. For instance, among the 35% of patients with a decline in strength, half of the patients had 4/5 strength in the weakest muscle tested. Furthermore, among patients without a preoperative motor deficit, the rate of postoperative motor decline decreased from 35% to 27% when the patients were followed for longer than 6 months. This finding supports a gradual improvement in motor function that is likely to occur over time and is consistent with clinical experience. These factors likely account for the lack of a difference in motor outcomes between patients with benign versus malignant brachial plexus tumors. Other groups have shown the rates of postoperative decline to be lower among patients with non-malignant tumors. For example, in our earlier series, the rates of motor decline for schwannoma, neurofibroma, and MPNST resection were 22%, 27%, and 64%, respectively.[
In addition to motor score, pain, and sensory function, several additional outcomes were examined. Serious complications were infrequent in our series and in related studies. We experienced vascular injury at a low rate comparable to Binder et al. in which one subclavian artery injury occurred during tumor mobilization in a series of 25 primary brachial plexus tumor cases.[
This study has several limitations. First, the study is retrospective in nature and follow-up is limited. For instance, some patients with MPNSTs are listed as not having recurred or received adjuvant therapy, which is due to lack of long-term follow-up for all patients. Due to the high number of patients included over a 15-year period, additional prospective follow-up was not feasible. Furthermore, survival analysis was not included for this reason. Whereas we included all tumors of the brachial plexus region to be as inclusive as possible and to increase the number of tumors included for analysis, the broad inclusion criteria may also be viewed as a limitation in that several outcome measures represent pooled findings for various tumor pathologies. Whenever the number of cases permitted, subgroup analyses were performed. Similar to other case series, our study focused on motor, pain, and sensory outcomes in relation to preoperative status; future studies will include patient-reported quality of life measures as well.
CONCLUSION
We describe a series of 103 brachial plexus region tumors operated on by a single surgeon at a single institution over a 15-year period with a special emphasis on motor and pain outcomes and a comparison with similar series over time. Comparable overall trends were noted between case series over time, with a palpable mass and associated tenderness being the most common presentation. Schwannoma was the most common tumor pathology in our study. For prognostic purposes, motor strength was unlikely to change following surgery. Those without preoperative motor deficits had a higher chance of a decline in motor strength after surgery, although this rate decreased over time. Conversely, the presence of a preoperative motor deficit was predictive of motor improvement after surgery.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Binder DK, Smith JS, Barbaro NM. Primary brachial plexus tumors: Imaging, surgical, and pathological findings in 25 patients. Neurosurg Focus. 2004. 16: E11
2. Das S, Ganju A, Tiel RL, Kline DG. Tumors of the brachial plexus. Neurosurg Focus. 2007. 22: E26
3. Desai KI. Primary benign brachial plexus tumors: An experience of 115 operated cases. Neurosurgery. 2012. 70: 220-33
4. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986. 57: 2006-21
5. Ganju A, Roosen N, Kline DG, Tiel RL. Outcomes in a consecutive series of 111 surgically treated plexal tumors: A review of the experience at the Louisiana State University Health Sciences Center. J Neurosurg. 2001. 95: 51-60
6. Go MH, Kim SH, Cho KH. Brachial plexus tumors in a consecutive series of twenty one patients. J Korean Neurosurg Soc. 2012. 52: 138-43
7. Huang JH, Samadani U, Zager EL. Brachial plexus region tumors: A review of their history, classification, surgical management, and outcomes. Neurosurg Q. 2003. 13: 151-61
8. Huang JH, Samadani U, Zager EL. Case studies for illustration and discussion: Peripheral nerve tumors. Neurosurg Clin N Am. 2004. 15: 241-9
9. Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol. 2004. 61: 372-8
10. Jia X, Yang J, Chen L, Yu C, Kondo T. Primary brachial plexus tumors: Clinical experiences of 143 cases. Clin Neurol Neurosurg. 2016. 148: 91-5
11. Jung IH, Yoon KW, Kim YJ, Lee SK. Analysis according to characteristics of 18 cases of brachial plexus tumors: A review of surgical treatment experience. J Korean Neurosurg Soc. 2018. 61: 625-32
12. Kim DH, Cho YJ, Tiel RL, Kline DG. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg. 2003. 98: 1005-16
13. Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 146 peripheral non-neural sheath nerve tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005. 102: 256-66
14. Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005. 102: 246-55
15. Lusk MD, Kline DG, Garcia CA. Tumors of the brachial plexus. Neurosurgery. 1987. 21: 439-53
16. Millan G, Casal D. Brachial plexus tumors in a tertiary referral center: A case series and literature review. Acta Reumatol Port. 2015. 40: 372-7
17. Ranalli NJ, Huang JH, Lee EB, Zhang PJ, Siegelman ES, Zager EL. Hemangiomas of the brachial plexus: A case series. Neurosurgery. 2009. 65: A181-8
18. Siqueira MG, Martins RS, Teixeira MJ. Management of brachial plexus region tumours and tumour-like conditions: Relevant diagnostic and surgical features in a consecutive series of eighteen patients. Acta Neurochir (Wien). 2009. 151: 1089-98