- Department of Neurosurgery, King Khalid Hospital, Hail, Saudi Arabia
- College of Medicine, University of Hail, Hail, Saudi Arabia
- Department of Neurosurgery, King Abdulaziz Medical City – National Guard Health Affairs, Riyadh, Saudi Arabia.
Correspondence Address:
Khalid Abdulkarim Alshammari, College of Medicine, University of Hail, Hail, Saudi Arabia.
DOI:10.25259/SNI_680_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ghaleb Shihadah Almesedin1, Hanan Odah Alshmaily2, Khalid Abdulkarim Alshammari2, Reem Sultan Albalawi3. Two case reports of Glanzmann thrombasthenia with intracranial hemorrhage and a review of the literature. 29-Dec-2023;14:448
How to cite this URL: Ghaleb Shihadah Almesedin1, Hanan Odah Alshmaily2, Khalid Abdulkarim Alshammari2, Reem Sultan Albalawi3. Two case reports of Glanzmann thrombasthenia with intracranial hemorrhage and a review of the literature. 29-Dec-2023;14:448. Available from: https://surgicalneurologyint.com/surgicalint-articles/two-case-reports-of-glanzmann-thrombocytopenia-with-intracranial-hemorrhage-and-a-review-of-the-literature/
Abstract
Background: Glanzmann’s thrombasthenia (GT) is a rare autosomal recessive disorder characterized by impaired platelet function. Symptoms range from mild to life-threatening bleeding. However, it is extremely rare for a patient to have intracranial bleeding. This study presents two cases of GT: one with a spontaneous epidural hematoma (EDH) and the other with a subarachnoid hemorrhage due to traumatic causes. The discussion that follows then derives relevant supporting insights through a review of the literature.
Case Description: Case Report 1: A 9-year-old girl with a known case of GT presented to an emergency department with a severe headache but no other complaints or history of trauma. The physical examination was normal. Computed tomography (CT) head without contrast revealed multiple EDHs with no midline shift. She received factor VII, tranexamic acid, and platelets transfusion and was admitted to the intensive care unit to be managed conservatively. After a month, a CT head follow-up showed complete resolution of all hematomas. Case Report 2: A 20-year-old male with a known case of GT was brought to the hospital with a history of loss of consciousness for several minutes after a road traffic accident. He suffered from a headache on regaining consciousness and received analgesia. CT head showed diffuse subarachnoid hemorrhage. He was managed with factor VII, tranexamic acid, and platelets transfusion and was admitted to an intermediate care unit for close observation.
Conclusion: In a GT patient with intracranial hemorrhage, conservative management with close clinical observation and platelet transfusion in combination with recombinant activated factor VII and/or antifibrinolytics can be safely conducted.
Keywords: Case report, Epidural bleeding, Epidural hematoma, Glanzmann thrombasthenia, Subarachnoid hemorrhage
INTRODUCTION
In 1918, Dr. Eduard Glanzmann was the first to document a case of Glanzmann’s thrombasthenia (GT). He described a platelet abnormality with defective clot retraction and an abnormal appearance on stained film.[
CASE REPORT 1
A 9-year-old Saudi female presented to the Emergency Department (ED) of the Maternity and Children Hospital in our city, complaining of a severe headache and epigastric pain that had lasted for one week. The patient’s history was mainly obtained from her mother. The headache was left unilateral, throbbing in nature, with intermittent attacks that responded partially to analgesia. It was preceded by photophobia, nausea, and vomiting. There was no history of head trauma, fever, neck pain, loss of consciousness, fits, neurological deficits, bruises, or bleeding from any body parts. This was a recurrent admission for the same complaint over the past six months. One month earlier, the patient was admitted to the Maternity and Children Hospital in Dammam City with the same complaint, which was diagnosed as a migraine. The patient has GT and shunted hydrocephalus since age one. Two of her younger siblings have GT, too. She also has helicobacter pylori gastritis on triple therapy. On examination, the patient was alert, conscious, oriented, and vitally stable. Her Glasgow coma scale (GCS) was 15/15. She looked ill and in pain. Pupils were round, regular, and reacting to light. There was no focal neurological defect. The ventriculoperitoneal shunt was working well with no signs of over-drainage. Other systems examination was unremarkable.
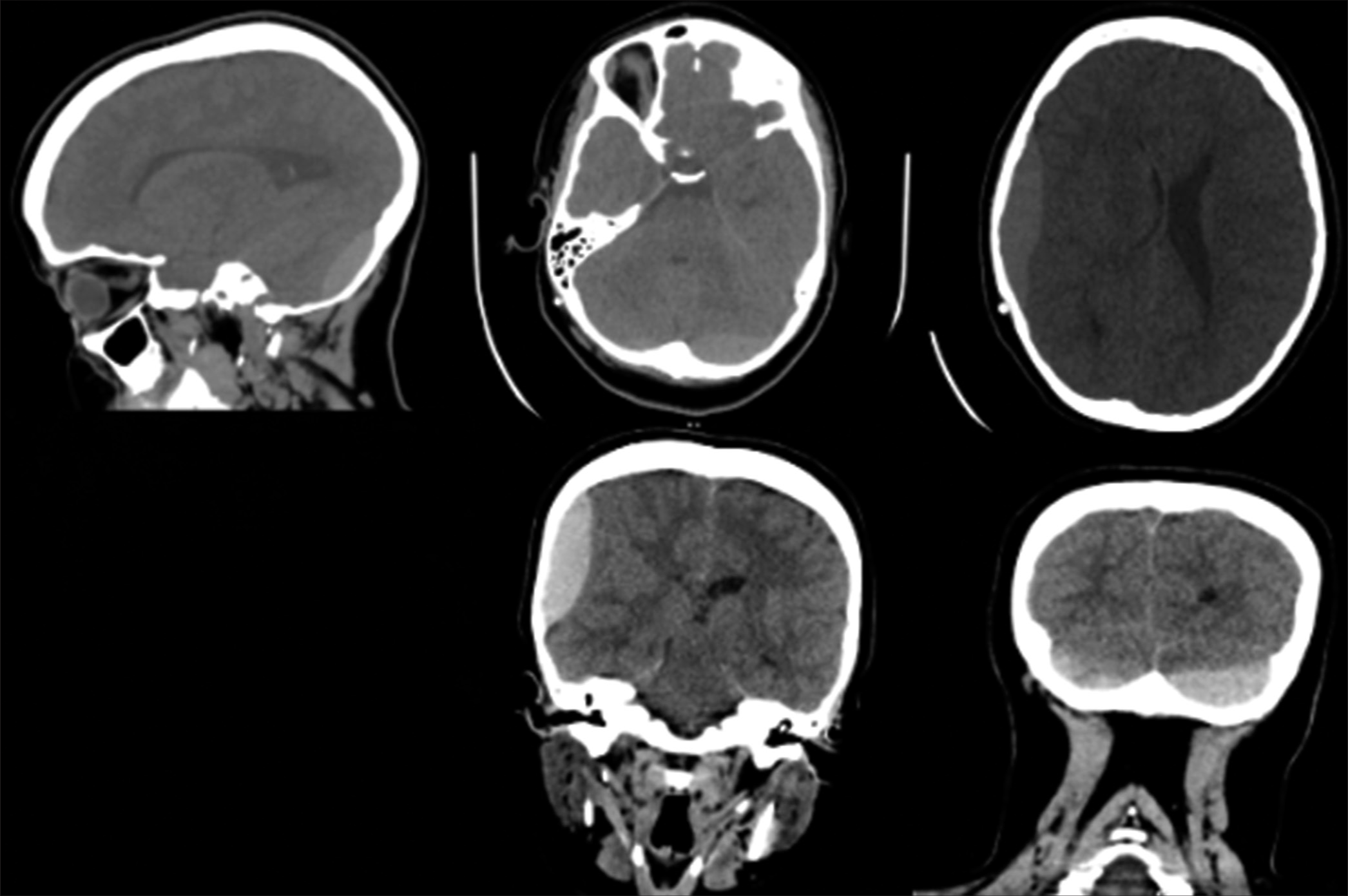
In the ED, she was given IV analgesics and proton pump inhibitors. Laboratory investigations were ordered. The results were as follows: hemoglobin 9.40 g/dL (N: 11.9–15), platelets count 442 × 10^16/mL (N:150–450), prothrombin time (PT) 7.50 (N:10.1–15), partial thromboplastin time (PTT) 25.40 (N: 26–40), and international-normalized ratio (INR) 0.58 (N: 0.8–1.2). Other findings were unremarkable. The patient underwent computed tomography (CT) of the brain, which revealed multiple biconvex hyperdense collections in the concavity of the skull bone, suggestive of acute epidural hematoma (EDH) [
Figure 1:
Initial computed tomography brain showing multiple biconvex hyperdense collection in the concavity of the skull bone, the largest in the right parietotemporal region measures 5.9 × 1.8 × 6.4m. Effacement of the right lateral ventricle with mild midline shift to the left with corresponding dilatation of the left lateral ventricle.
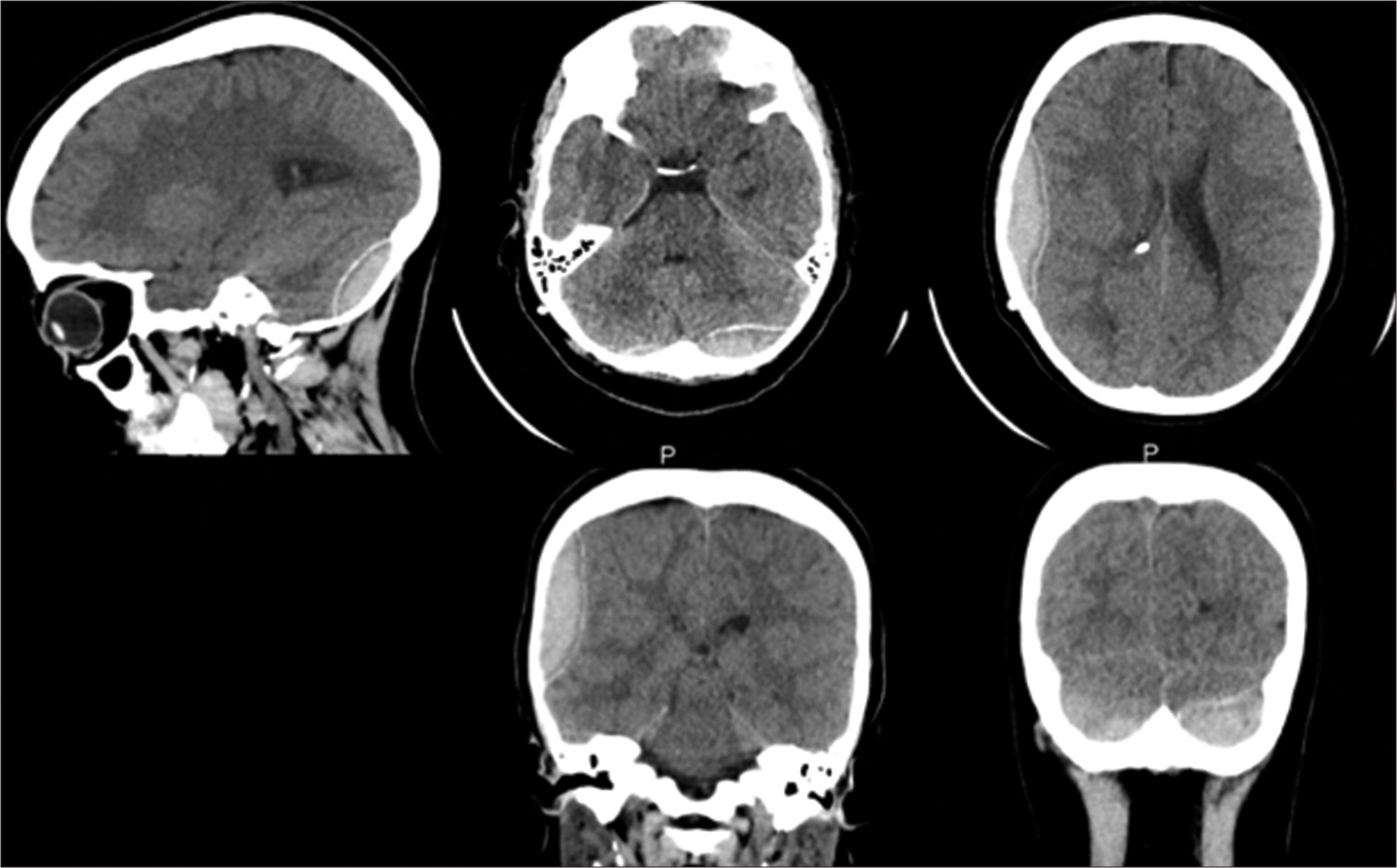
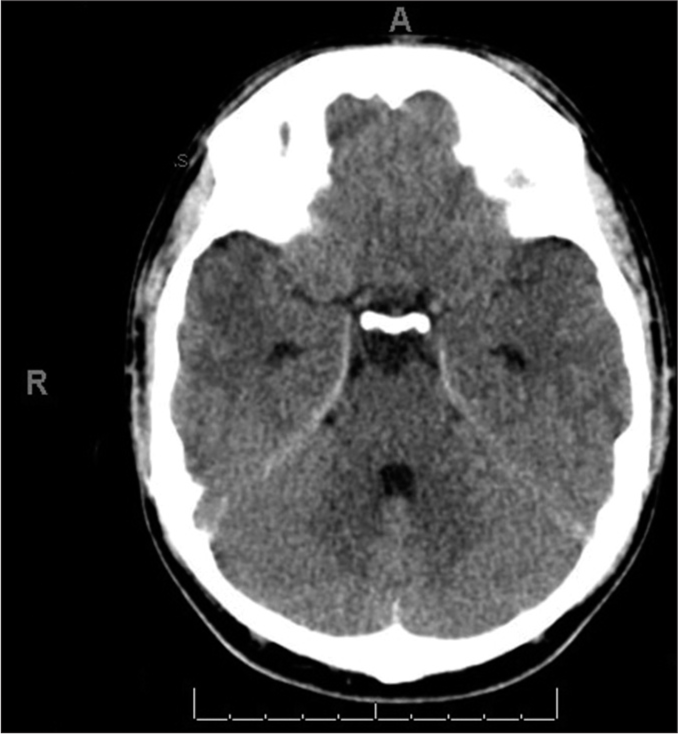
On day 10 of her admission, the patient was doing well. She was conscious-oriented, with a GCS of 15/15 and stable vital signs. The headache had resolved, and there was no vomiting or neurological deficit. A fifth CT brain was done [
CASE REPORT 2
A 20-year-old Saudi male came to the hospital via ambulance with a brief history of loss of consciousness after a road traffic accident that involved a traumatic brain injury. He was a backseat passenger whose head hit the window and experienced a severe headache after regaining consciousness. The patient did not complain of nausea, vomiting, convulsion, vision disturbances, slurred speech, or external bleeding. He is a non-smoker, a son of a consanguineous marriage, and a known case of GT. On physical examination, the patient was vitally stable, conscious, and oriented. His GCS was 15/15. His pupils were round, regular, and reacting to the light. There was no external bleeding, but there was purpuric eruption over his neck, upper and lower extremities, and a large hematoma over his right thigh.
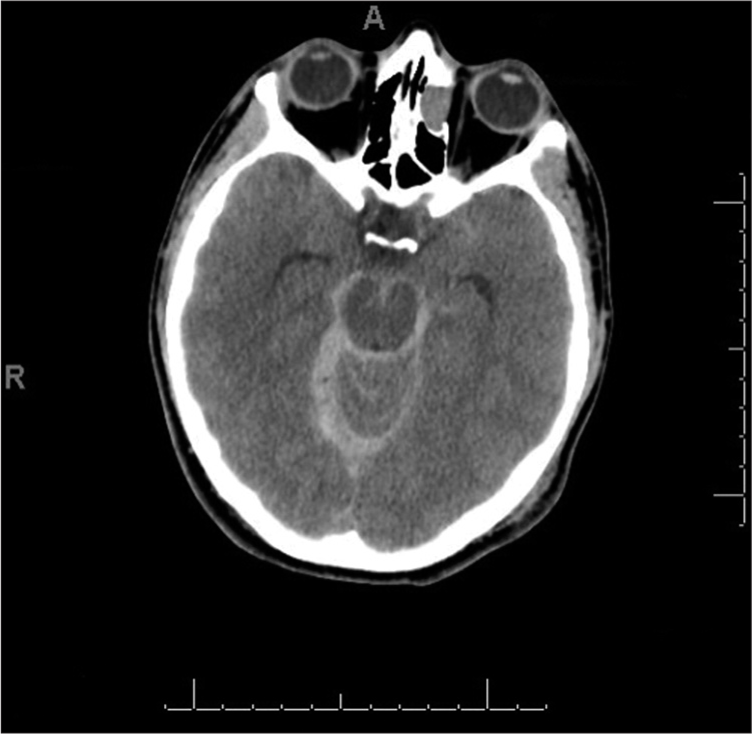
There were no neurological deficits, and the other systems examination was unremarkable. Laboratory investigation revealed hemoglobin 14.60 q/dL, platelets 201 × 10^3/micr, PT 12.50 s, PTT 32.70, and INR 1.10. Other findings were unremarkable. A CT brain revealed a bilateral subarachnoid hemorrhage that could be noted mainly at the basal cistern along the cerebellar tentorium and the high right occipital cortical sulci. It also showed a left high parietal extracranial subgaleal hematoma alongside a right occipital extracranial subgaleal hematoma. There was no midline structure shift, and the ventricular system was normal, with no signs or evidence of an underlying vascular pathology. The patient was given IV analgesia for the headache. The plan was to give 500 mg tranexamic acid; factor VII concentrate 90 mg/kg, and six units of platelets transfusion after a hematologist consultation [
Figure 4:
Computed tomography brain showing a bilateral subarachnoid hemorrhage that could be noted mainly at basal cistern along cerebellar tentorium and high right occipital cortical sulci and a left high parietal extracranial subgaleal hematoma in addition to a right occipital extracranial subgaleal hematoma.
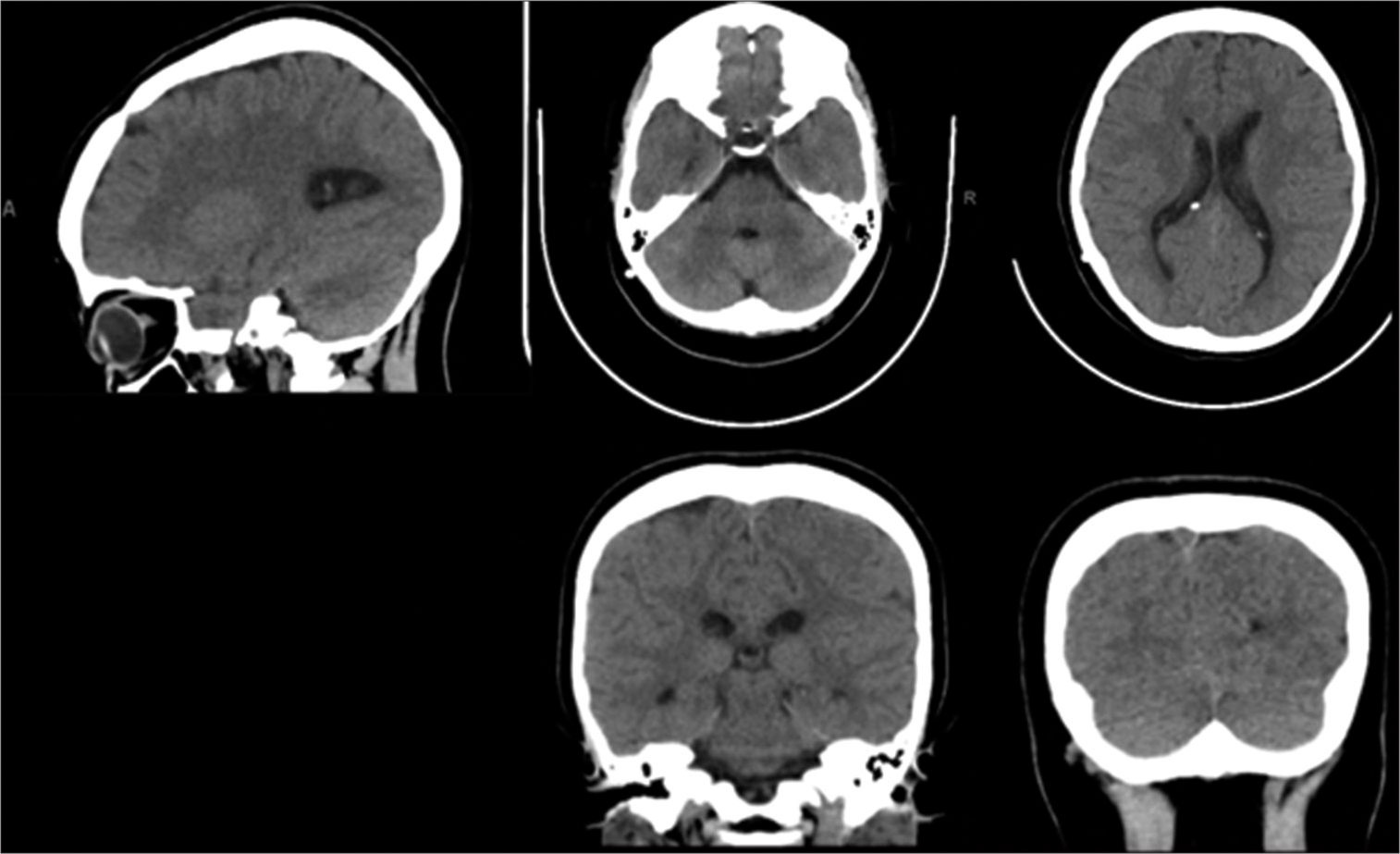
The neurosurgery team has planned a conservative management approach. The patient was admitted to an intermediate care unit, with close monitoring of vitals, consciousness level, and pupil size, alongside further laboratory investigation, a follow-up CT brain after 24 h, and to continue with the hematologist’s plan. In addition, the team was notified if there was any deterioration to require an urgent CT brain and to inform the on-call neurosurgeon. On the 2nd day, the patient was vitally stable, with no change in consciousness level, no neurological deficits, nor changes in pupil size. There were no new complaints except for a headache, for which he received a 1 g perfalgan injection, to be given every six hours as necessary. A follow-up CT brain, when compared with the one done at admission, showed no appreciable time interval changes. Three days later, the patient was moved to a common room with continuity of the same management. After ten days of observation, the patient’s condition was stable, and he was doing well, with no new complaints. A CT head was obtained [
DISCUSSION
GT is a rare autosomal recessive disorder characterized by impaired platelet function or a severe reduction in platelet aggregation due to deficiency or abnormality of platelet GP GPIlb and/or GPIlla receptors. Certain ethnic groups with higher rates of consanguinity, such as Indians, Iranians, Iraqi Jews, Palestinian and Jordanian Arabs, and French Gypsies, are more prone to GT.[
A literature search for cases of CNS bleeding in patients with GT yielded six studies, four of which included intracranial bleeding, and two were in the spinal cord.[
CONCLUSION
GT is a rare inherited blood disorder, with spontaneous intracranial hemorrhage is an even rarer presentation of this condition, which can nonetheless be life-threatening. The reviewed cases showed that, provided the patient’s level of consciousness is intact. Serial CT brain images do not reveal any change in the size of the hematoma; conservative management with close clinical observation and platelets transfusion in combination with recombinant activated factor VII and/or antifibrinolytics can be safely conducted in a GT patient with intracranial hemorrhage. The outcome was excellent and saved the patient from the risks of surgical intervention.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. AI-Barghouthi SK, AI-Othman A, Lardhi A. Glanzmann’s thrombasthenia-spectrum of clinical presentation on Saudi patients in the eastern province. J Family Community Med. 1997. 4: 57
2. Al Barbarawi MM, Allouh MZ, Audat ZA, Aleshawi AJ, Al-Shari OM. Unique case of successful surgical treatment of recurrent spinal epidural hematoma after lumbar disc surgery in a Glanzmann thrombasthenia patient. Br J Neurosurg. 2019. 37: 853-5
3. Al-Abdulkareem AA, Ballal SG. Consanguineous marriage in an urban area of Saudi Arabia: Rates and adverse health effects on the offspring. J Community Health. 1998. 23: 75-83
4. Al-Fawaz IM, Gader AM, Bahakim HM, Al-Mohareb F, Al-Momen AK, Harakati MS. Hereditary bleeding disorders in Riyadh, Saudi Arabia. Ann Saudi Med. 1996. 16: 257-61
5. Bell JA, Savidge GF. Glanzmann’s thrombasthenia proposed optimal management during surgery and delivery. Clin Appl Thrombosis/Hemostasis. 2003. 9: 167-70
6. Botero JP, Lee K, Branchford BR, Bray PF, Freson K, Lambert MP. Glanzmann thrombasthenia: Genetic basis and clinical correlates. Haematologica. 2020. 105: 888
7. Chakati V, Bukka DP, Erigaisi SR, Anchuri SS. Glanzmann thrombasthenia: A case report of a rare inherited coagulation disorder presenting with traumatic head injury. EMJ Hematol. 2021. 9: 110-3
8. Fernández-Castellano G, Mayorga-Buiza MJ, Gallego-Solano J, Vazquez-Rubio R, Rivero-Garvia M. Acute epidural hematoma following acute subdural hematoma evacuation in a child with Glanzmann thrombasthenia. J Neurosurg Anesthesiol. 2016. 28: 431-3
9. George J, Caen J, Nurden A. Glanzmann’s thrombasthenia: The spectrum of clinical disease. Blood. 1990. 75: 1383-95
10. Kannan M, Saxena R. Glanzmann’s thrombasthenia: An overview. Clin Appl Thromb Hemost. 2009. 15: 152-65
11. Kongalappa S, Reddy JM, Durugappa T, D’souza F, Subramanian S, Prakash A. Glanzmann thrombasthenia in children: Experience from a tertiary care center in southern India. J Pediatr Hematol Oncol. 2019. 41: E68-71
12. Lee SM, Kim KN, Kim SY. Perioperative hemostatic management of a pediatric patient with glanzmann thrombasthenia undergoing osteoplastic craniotomy and hematoma removal: A case report. Acta Haematol. 2019. 142: 244-8
13. Mahboub SM, Alsaqabi AA, Allwimi NA, Aleissa DN, AlMubarak BA. Prevalence and pattern of consanguineous marriage among educated married individuals in Riyadh. J Biosoc Sci. 2020. 52: 768-75
14. Nair S, Ghosh K, Kulkarni B, Shetty S, Mohanty D. Glanzmann’s thrombasthenia: Updated. Platelets. 2002. 13: 387-93
15. Sebastiano C, Bromberg M, Breen K, Hurford MT. Glanzmann’s thrombasthenia: Report of a case and review of the literature. Int J Clin Exp Pathol. 2010. 3: 443
16. Tabibian S, Motlagh H, Naderi M, Dorgalaleh A. Intracranial hemorrhage in congenital bleeding disorders. Blood Coagul Fibrinolysis. 2018. 29: 1-11
17. Yamahata H, Hirahara K, Tomosugi T, Yamada M, Ishii T, Uetsuhara K. Acute epidural hematoma in a patient with Glanzmann’s thrombasthenia: Case report. Neurol Med Chir (Tokyo). 2010. 50: 928-30