- Mark and Mary Stevens Neuroimaging and Informatics Institute, University of Southern California,
- Departments of Radiology, University of Southern California, Los Angeles, California, United States,
- Medicine, Division of Endocrinology, University of Southern California, Los Angeles, California, United States,
- Neurological Surgery, University of Southern California, Los Angeles, California, United States.
Correspondence Address:
Vishal Patel, Mark and Mary Stevens Neuroimaging and Informatics Institute, University of Southern California, Los Angeles, California, United States.
DOI:10.25259/SNI_787_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jonathan Lee1, Charles Li2, Chia-Shang J. Liu2, Mark Shiroishi2, John D. Carmichael3, Gabriel Zada4, Vishal Patel1,2. Ultra-high field 7 T MRI localizes regional brain volume recovery following corticotroph adenoma resection and hormonal remission in Cushing’s disease: A case series. 03-Jun-2022;13:239
How to cite this URL: Jonathan Lee1, Charles Li2, Chia-Shang J. Liu2, Mark Shiroishi2, John D. Carmichael3, Gabriel Zada4, Vishal Patel1,2. Ultra-high field 7 T MRI localizes regional brain volume recovery following corticotroph adenoma resection and hormonal remission in Cushing’s disease: A case series. 03-Jun-2022;13:239. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11628
Abstract
Background: Cushing’s disease (CD) is defined by glucocorticoid excess secondary to the increased section of corticotropin by a pituitary adenoma. Magnetic resonance imaging (MRI) studies performed at 1.5 or 3 Tesla (T) have demonstrated correlations between regional changes in brain structure and the progression of CD. In this report, we examine the changes in brain volume following corticotroph pituitary adenoma resection using ultra-high field 7 T MRI to increase the accuracy of our volumetric analyses.
Methods: Thirteen patients were referred to the endocrinology clinic at our institution from 2017 to 2020 with symptoms of cortisol excess and were diagnosed with ACTH-dependent endogenous Cushing syndrome. Five patients had follow-up 7 T imaging at varying time points after a transsphenoidal resection.
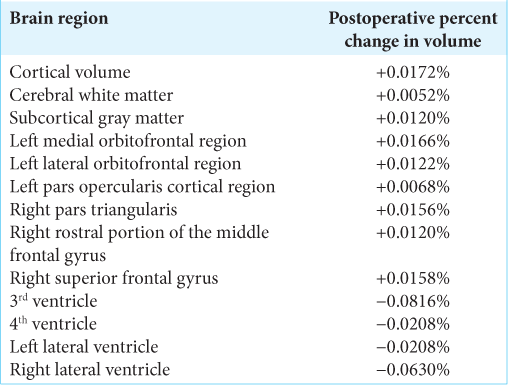
Results: Symmetrized percent change in regional volumes demonstrated a postoperative increase in cortical volume that was relatively larger than that of cerebral white matter or subcortical gray matter (percent changes = 0.0172%, 0.0052%, and 0.0120%, respectively). In the left cerebral hemisphere, the medial orbitofrontal, lateral orbitofrontal, and pars opercularis cortical regions experienced the most robust postoperative percent increases (percent changes = 0.0166%, 0.0122%, and 0.0068%, respectively). In the right cerebral hemisphere, the largest percent increases were observed in the pars triangularis, rostral portion of the middle frontal gyrus, and superior frontal gyrus (percent changes = 0.0156%, 0.0120%, and 0.0158%).
Conclusion: Cerebral volume recovery following pituitary adenoma resection is driven by changes in cortical thickness predominantly in the frontal lobe, while subcortical white and gray matter volumes increase more modestly.
Keywords: Brain volume, Cushing’s disease, Pituitary adenoma, Ultra-high field MRI
INTRODUCTION
Cushing’s disease (CD) is caused by a pituitary adenoma that secretes excess levels of adrenocorticotropic hormone (ACTH or corticotropin), which, in turn, stimulates the excess downstream release of cortisol. Supraphysiologic levels of cortisol from any cause result in a set of signs and symptoms that comprise the Cushing syndrome (CS), including characteristic fatty tissue deposits, purple striae, hirsutism, and fatigue, as well as reduced survival.[
Considering these associations, several investigators have analyzed cerebral morphometric parameters in relation to the progression and treatment of CD. One study of adult patients showed that there was an increase in white matter volume and a decrease in cortical thickness, mainly in the frontal and parietal lobes, of patients with persistent CS versus those who achieved endocrinological remission.[
To the best of our knowledge, all previous studies measuring cerebral atrophy in relation to CD have been conducted at conventional magnetic field strengths of 1.5 or 3 Tesla, which have poorer signal-to-noise and contrast-to-noise characteristics than UHF strength 7 T MRI. This difference is particularly important when examining fine structural features such as cortical thickness or pituitary microarchitecture. For example, it has been shown that up to 40% of patients with CD have adenomas that are not detectable by conventional MRI.[
The improved spatial and contrast resolution of UHF MRI has yet to be applied to the volumetric analysis of brain regions in the setting of CD recovery. High-resolution structural detail is especially important for volumetric studies, as errors in linear measurements become geometrically magnified, and this can result in reduced sensitivity or spurious conclusions. In this report, we present the first such analysis of regional brain volumes in a cohort of patients with CD who underwent high-resolution UHF MRI before and following transsphenoidal surgery.
MATERIALS AND METHODS
Subjects
Thirteen patients were referred to the endocrinology clinic at our institution with symptoms of cortisol excess. Following clinical and laboratory workup, each patient was diagnosed with ACTH-dependent endogenous CS according to societal guidelines, with concern for CD.[
MRI studies
The patients underwent 7 T MRI per previously published protocols, including whole-brain 0.7 mm isotropic resolution magnetization-prepared rapid gradient echo (MP-RAGE) sequences and postcontrast (0.2 mL/kg gadoterate meglumine) sellar sequences that identified focal hypoenhancing pituitary lesions in all cases.[
Volumetric and statistical analysis
To obtain quantitative measures for the differential recovery of different brain regions, we performed a volumetric analysis of the preoperative and postoperative 7 T MP-RAGE sequences using FreeSurfer’s longitudinal workflow and a two-stage model.[
The FreeSurfer workflow consists of three major steps.[
Figure 1:
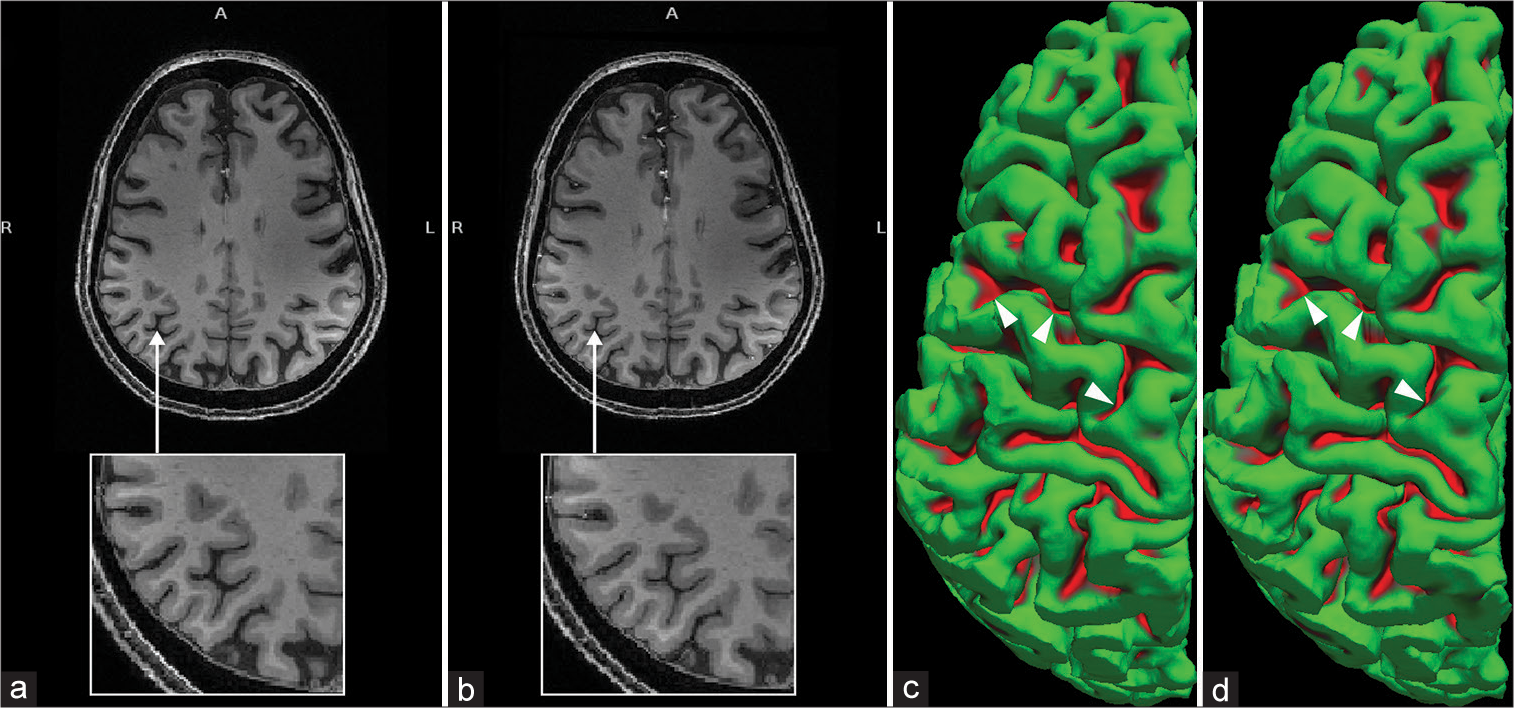
Postoperative cortical thickness recovery in patient 2. Matched T1-weighted axial slices from preoperative (a) and 81-day postoperative (b) 7 T MRI illustrates the expansion of the cortical gray matter with concordant narrowing of adjacent sulci, best seen on the magnified insets of the right parietal lobe (arrows). Superior views of 3D surface renderings of the right cerebral hemisphere generated from the same preoperative (c) and postoperative (d) 7 T MRIs also reveal narrowing of the frontoparietal sulci (red), best seen from this perspective along the precentral sulcus (arrowheads).
Figure 2:
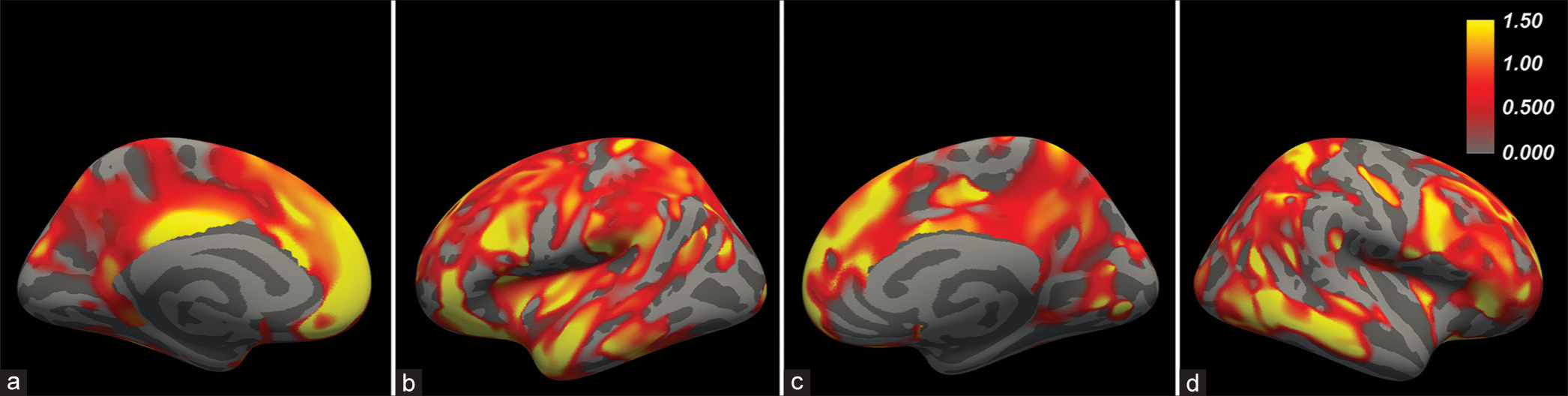
Regression results for postoperative increases in cortical thickness across all three subjects. Inflated cortical surface renderings are shown from the medial/lateral projections for the left (a and b) and right (c and d) hemispheres. In the overlaid color scale, golden hues indicate areas of greater postoperative cortical thickness increase, scaled as z-scores. The scale represents −log10 (p) values.
RESULTS
Both patients 2 and 3 underwent follow-up 7 T MRI at 81 days following surgery, while patients 1, 4, and 5 went through imaging at 882, 333, and 392 days following surgery, respectively. Direct visual comparison of the preoperative and postoperative scans revealed a clear reversal of cerebral atrophy, identifiable as increased cortical thickness and corresponding sulcal narrowing. A representative example of this phenomenon is provided in [
DISCUSSION
Imaging analysis
Our findings demonstrate recovery of frontotemporal cortical volume in CD patients following surgical treatment with UHF 7 T MRI. We are able to better depict postoperative percentage increases with better spatial resolution. Two commonly used standards for gray matter analysis are cortical thickness and surface area. Thickness is a measure of neuron and glia size, number, and arrangement in specific brain regions, while surface area is associated with the number of columns in a region of interest. The data collected using 7 T MRI and analysis pipelines such as FreeSurfer in this work have shown that the frontotemporal areas of the brain may increase in cortical thickness and volume after CD surgical treatment and hormonal remission.[
Literature review
CD is an important disease model for understanding the effect of cortisol on brain structures.[
The volume changes in the brain after a transsphenoidal resection may reflect a reversal of damage in neuronal and nonneuronal glial cells in the affected areas, which may be sensitive to glucocorticoid (GC) excess. Animal models with GC excess have been shown to have a significant loss of synapses on pyramidal cells of hippocampal region CA3 and structural changes in afferent mossy fibers terminating on those neurons.[
Limitations
Due to the limited study sample of five patients, the observed volume changes are preliminary and will require follow-up studies to assess the time course of frontoparietal volumetric changes. Further follow-up with a larger-scale analysis is needed to make a more statistically significant call for the frontoparietal changes as seen in the data here. In this specific study, patients 2 and 3 had their follow-up MRI both at 81 days after surgery, which is an order of magnitude shorter than the other three patients’ follow-up MRI. The changes in the brain for all five patients were still consistent with each other, so there may be a plateau in the parameter changes during the posttreatment period. Nonetheless, additional UHF scans at defined and consistent intervals after the patient’s operations are an area of interest since some areas of the brain may recover more quickly than others. In addition, set intervals will show a more comprehensive timeline of the relief of CD symptoms in correlation with brain parameter changes.
Cognitive and psychiatric changes were not evaluated in our sample of five patients. Cognitive difficulties and depression can persist in patient’s following pituitary resections, even when brain volumes have normalized.[
AI
The diagnosis and management of CD can be challenging since some patients may have an atypical presentation that overlaps with other metabolic syndromes. Thus, an AI platform could be useful to isolate features that are characteristic of preoperative and postoperative CD patients and to better determine when patients are in full remission of the disease.[
CONCLUSION
UHF MRI yields observable increases in cortical thickness and overall brain volume following corticotroph adenoma resection and achievement of hormonal remission in patients with Cushing disease. This cerebral volume recovery appears predominantly to involve the cortical gray matter compartment with a frontotemporal predominance. This proof-of-concept work highlights the sensitivity and accuracy of the 7 T technique, which may be applied in future, large-scale studies to elucidate the mechanisms underlying the cognitive and psychiatric symptoms associated with CD. In combination with AI tools, UHF imaging techniques have a high potential to analyze microlevel structural differences associated with certain neurological disorders consistently.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alsumali A, Cote DJ, Regestein QR, Crocker E, Alzarea A, Zaidi HA. The impact of transsphenoidal surgery on neurocognitive function: A systematic review. J Clin Neurosci. 2017. 42: 1-6
2. Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A. Cushing’s syndrome causes irreversible effects on the human brain: A systematic review of structural and functional MRI studies. Eur J Endocrinol. 2017. 173: R1-14
3. Andela CD, van der Werff SJ, Pannekoek JN, van den Berg SM, Meijer OC, van Buchem MA. Smaller grey matter volumes in the anterior cingulate cortex and greater cerebellar volumes in patients with long-term remission of Cushing’s disease: A case-control study. Eur J Endocrinol. 2013. 169: 811-9
4. Bourdeau I, Bard C, Noël B, Leclerc I, Cordeau MP, Bélair M. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab. 2002. 87: 1949-54
5. Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997. 10: 171-8
6. Crespo I, Esther GM, Santos A, Valassi E, Yolanda VG, De Juan-Delago M. Impaired decision-making and selective cortical frontal thinning in Cushing’s syndrome. Clin Endocrinol (Oxf). 2014. 81: 826-33
7. de Rotte AA, Groenewegen A, Rutgers DR, Witkamp T, Zelissen PM, Meijer FJ. High resolution pituitary gland MRI at 7.0 tesla: A clinical evaluation in Cushing’s disease. Eur Radiol. 2016. 26: 271-7
8. Devoe DJ, Miller WL, Conte FA, Kaplan SL, Grumbach MM, Rosenthal SM. Long-term outcome in children and adolescents after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab. 1997. 82: 3196-202
9. Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab. 1997. 82: 912-9
10. Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006. 11: 2746-58
11. Hinojosa-Amaya JM, Cuevas-Ramos D. The definition of remission and recurrence of Cushing’s disease. Best Pract Res Clin Endocrinol Metab. 2021. 35: 101485
12. Hou B, Gao L, Shi L, Luo Y, Guo X, Young GS. Reversibility of impaired brain structures after transsphenoidal surgery in Cushing’s disease: A longitudinal study based on an artificial intelligence-assisted tool. J Neurosurg. 2020. 1: 1-10
13. Jiang H, Ren J, He NY, Liu C, Sun YH, Jian FF. Volumetric magnetic resonance imaging analysis in patients with short-term remission of Cushing’s disease. Clin Endocrinol (Oxf). 2017. 87: 367-74
14. Keil MF, Merke DP, Gandhi R, Wiggs EA, Obunse K, Stratakis CA. Quality of life in children and adolescents 1-year after cure of Cushing syndrome: A prospective study. Clin Endocrinol (Oxf). 2009. 71: 326-33
15. Keil MF, Zametkin A, Ryder C, Lodish M, Stratakis CA. Cases of psychiatric morbidity in pediatric patients after remission of cushing syndrome. Pediatrics. 2016. 137: e20152234
16. Kelly WF, Kelly MJ, Faragher B. A prospective study of psychiatric and psychological aspects of Cushing’s syndrome. Clin Endocrinol (Oxf). 1996. 45: 715-20
17. Khiat A, Bard C, Lacroix A, Boulanger Y. Recovery of the brain choline level in treated Cushing’s patients as monitored by proton magnetic resonance spectroscopy. Brain Res. 2000. 862: 301-7
18. Laws ER, Pace L.editors. Cushing’s Disease: An often Misdiagnosed and not so Rare Disorder. Cambridge, Massachusetts: Academic Press; 2016. p.
19. Lonser RR, Nieman L, Oldfield EH. Cushing’s disease: Pathobiology, diagnosis, and management. J Neurosurg. 2017. 126: 404-17
20. Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS. Altered amygdala and hippocampus function in adolescents with hypercortisolemia: A functional magnetic resonance imaging study of Cushing syndrome. Dev Psychopathol. 2008. 20: 1177-89
21. McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995. 5: 205-16
22. Merke DP, Giedd JN, Keil MF, Mehlinger SL, Wiggs EA, Holzer S. Children experience cognitive decline despite reversal of brain atrophy one year after resolution of Cushing syndrome. J Clin Endocrinol Metab. 2005. 90: 2531-6
23. Moeskops P, Viergever MA, Mendrik AM, de Vries LS, Benders MJ, Isgum I. Automatic segmentation of MR brain images with a convolutional neural network. IEEE Trans Med Imaging. 2016. 35: 1252-61
24. Molitch ME. Diagnosis and treatment of pituitary adenomas: A review. JAMA. 2017. 317: 516-24
25. Momose KJ, Kjellberg RN, Kliman B. High incidence of cortical atrophy of the cerebral and cerebellar hemispheres in Cushing’s disease. Radiology. 1971. 99: 341-8
26. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006. 367: 1605-17
27. Ohara N, Suzuki H, Suzuki A, Kaneko M, Ishizawa M, Furukawa K. Reversible brain atrophy and cognitive impairment in an adolescent Japanese patient with primary adrenal Cushing’s syndrome. Neuropsychiatr Dis Treat. 2014. 10: 1763-7
28. Patel V, Liu CJ, Shiroishi MS, Hurth K, Carmichael JD, Zada G. Ultra-high field magnetic resonance imaging for localization of corticotropin-secreting pituitary adenomas. Neuroradiology. 2020. 62: 1051-4
29. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016. 4: 611-29
30. Ragnarsson O, Berglund P, Eder DN, Johannsson G. Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J Clin Endocrinol Metab. 2012. 97: E1640-8
31. Resmini E, Santos A, Gómez-Anson B, Vives Y, Pires P, Crespo I. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012. 97: 663-71
32. Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012. 61: 1402-18
33. Simmons NE, Do HM, Lipper MH, Laws ER. Cerebral atrophy in Cushing’s disease. Surg Neurol. 2000. 53: 72-6
34. Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med. 2001. 63: 985-93
35. Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol Psychiatry. 1999. 46: 1595-602
36. Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003. 53: 233-8
37. Starkman MN, Schteingart DE, Schork MA. Depressed mood and other psychiatric manifestations of Cushing’s syndrome: Relationship to hormone levels. Psychosom Med. 1981. 43: 3-18
38. Stroud A, Dhaliwal P, Alvarado R, Winder MJ, Jonker BP, Grayson JW. Outcomes of pituitary surgery for Cushing’s disease: A systematic review and meta-analysis. Pituitary. 2020. 23: 595-609
39. Tiemensma J, Kokshoorn NE, Biermasz NR, Keijser BJ, Wassenaar MJ, Middelkoop HA. Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J Clin Endocrinol Metab. 2010. 95: 2699-714
40. Tirosh A, RaviPrakash H, Papadakis GZ, Tatsi C, Belyavskaya E, Charalampos L. Computerized analysis of brain MRI parameter dynamics in young patients with cushing syndrome a case-control study. J Clin Endocrinol Metab. 2020. 105: dgz303
41. Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009. 45: S173-86