- Department of Neurosurgery and Radiotherapy, Hospital do Coracao (HCOR Neurosciences), Gamma Knife,
- Departament of Psychiatry, Faculty of Medicine, University of São Paulo, São Paulo, Brazil.
Correspondence Address:
Antonio A. F. De Salles
Department of Neurosurgery and Radiotherapy, Hospital do Coracao (HCOR Neurosciences), Gamma Knife,
Departament of Psychiatry, Faculty of Medicine, University of São Paulo, São Paulo, Brazil.
DOI:10.25259/SNI-65-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bruno Fernandes de Oliveira Santos, Alessandra Gorgulho, Crystian W. C. Saraiva, Antonio Carlos Lopes, João Gabriel Ribeiro Gomes, Anderson M. Pássaro, Marcelo Q. Hoexter, Eurípedes C. Miguel, Antonio A. F. De Salles. Understanding gamma ventral capsulotomy: Potential implications of diffusion tensor image tractography on target selectivity. 05-Jul-2019;10:136
How to cite this URL: Bruno Fernandes de Oliveira Santos, Alessandra Gorgulho, Crystian W. C. Saraiva, Antonio Carlos Lopes, João Gabriel Ribeiro Gomes, Anderson M. Pássaro, Marcelo Q. Hoexter, Eurípedes C. Miguel, Antonio A. F. De Salles. Understanding gamma ventral capsulotomy: Potential implications of diffusion tensor image tractography on target selectivity. 05-Jul-2019;10:136. Available from: http://surgicalneurologyint.com/surgicalint-articles/9459/
Abstract
Background: The role of tractography in gamma ventral capsulotomy (GVC) planning is still unclear. This paper aims to describe the spatial distribution of medial orbitofrontal cortex (OFC) and lateral OFC fibers passing through the anterior limb of the internal capsule (ALIC) and analyze quantitative tractography parameters that differentiate obsessive-compulsive disorder (OCD) individuals from other neurosurgery functional patients (morbid obesity and Parkinson’s disease [PD]).
Methods: Twenty patients undergoing functional stereotactic procedures, between 2013 and 2016, were included in this study. OCD patients underwent GVC (single shot 150 Gy and 4-mm collimators). PD and morbid obesity patients were submitted to deep brain stimulation implants. Diffusion tensor image tractography was reconstructed using Brainlab Elements software (Brainlab AG, Munich, Germany).
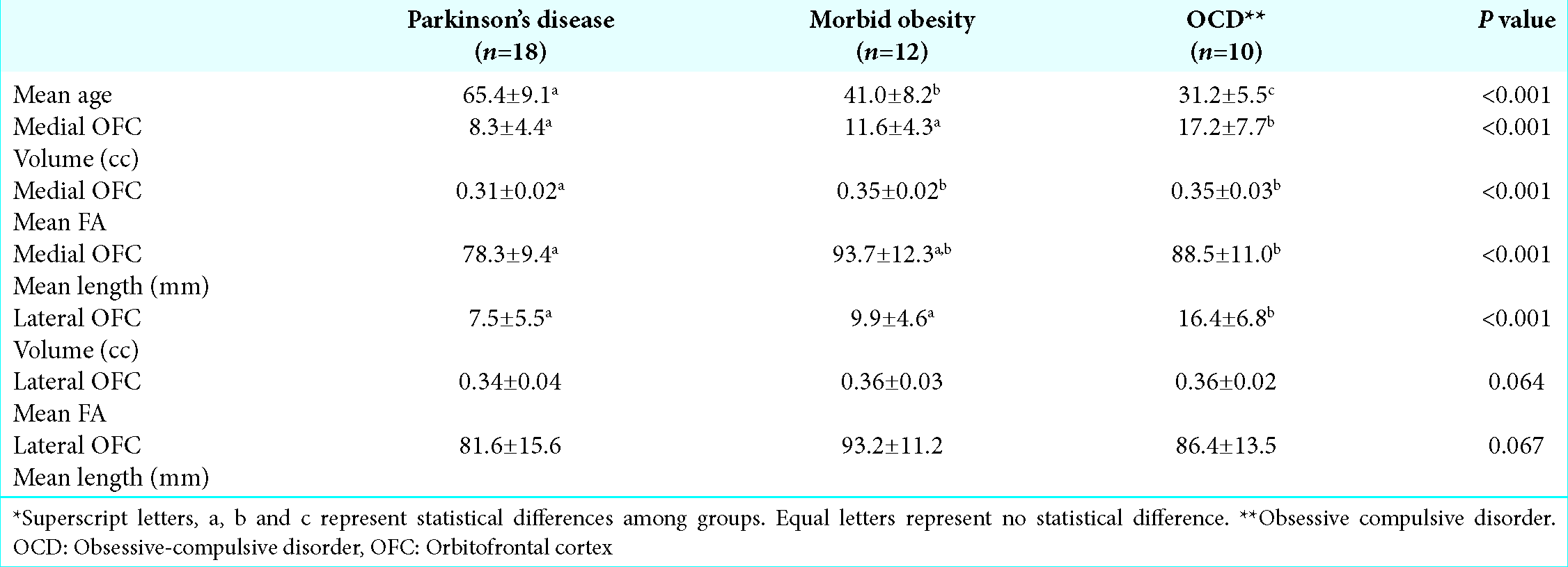
Results: Nine PD, six morbid obesity, and five OCD patients were included with a mean age of 65.4 ± 9.1, 41.0 ± 8.2, and 31.2 ± 5.5, respectively, which are statistically different from each other (P P
Conclusions: Medial and lateral OFC tract fibers have a general standard distribution in the anterior internal capsule (lateral OFC higher than medial OFC fibers). There are differences between obesity, Parkinson, and OCD patients regarding fiber tract statistics.
Keywords: Capsulotomy, Functional stereotactic neurosurgery, Gamma knife, Obsessive-compulsive disorder, Tractography and anisotropy
INTRODUCTION
Obsessive-compulsive disorder (OCD), characterized by obsessional thoughts and compulsive rituals, is one of the most prevalent psychiatric disorders, affecting 2% of the population worldwide.[
Ablative lesions at the anterior limb of the internal capsule (ALIC) can also be done using Gamma Knife radiosurgery. Gamma Knife capsulotomy has evolved to smaller internal capsule lesions, in an effort to minimize side effects. The trend has also been toward decreasing the radiation dose and targeting the most ventral fibers of internal capsule (gamma ventral capsulotomy [GVC]).[
GVC promotes a stereotactic lesion at the ALIC based on anatomic identification alone.[
Both GVC and DBS attempt to modify pathological activity in the fiber tracts between orbitofrontal cortex (OFC) and thalamus (hyperactive orbito-fronto-striato-thalamo-cortical circuitry) by targeting the ventral capsule/ventral striatum area and nucleus accumbens.[
The objective of this study is to describe the spatial distribution of medial and lateral OFC fibers passing through the anterior internal capsule and analyze quantitative tractography parameters that differentiate OCD individuals from other functional neurosurgical patients (morbid obesity and Parkinson’s disease [PD]).
SUBJECTS AND METHODS
Patients
Twenty patients undergoing functional stereotactic procedures, between 2013 and 2016, under magnetic resonance imaging (MRI) guidance in our institution were studied. Our Institutional Review Board approved the study. The State Review Board of the state of Sao Paulo, Brazil, approved the GVC procedure for all patients in this study. Patients underwent surgery for different diagnoses, including OCD (n = 5), PD (n = 9), and morbid obesity (n = 6). Patients were submitted to DBS implants in the subthalamic nucleus, hypothalamus, and globus pallidus pars interna (GPi), as well as GVC.
MRI acquisition
MRI was obtained days before or at the day of the procedures, without a stereotactic frame, to avoid artifacts. Anatomical and DTI were performed on a GE 1.5 Tesla MRI system with an eight-channel head coil with the following technical specifications: gradient of 40 mT/m, matrix of 256 × 256 pixels, and field of view (FOV) of 256 x 256 mm. The FLAIR sequence was acquired in the sagittal plane with a repetition time (TR) of 6000 ms, echo time (TE) of 353 ms, and inversion time of 2200. The T1 sequence with gadolinium was acquired in the sagittal plane with TR of 2000 ms and TE of 3.42 ms. DTI data were acquired using dual spin-echo echo-planar imaging with TR =11,500 ms, TE = 88.6–209 ms (optimized), acquisition matrix = 254 mm×254 mm, and FOV = 250 mm. A slice thickness of 3 mm with no gap was used. Diffusion-sensitizing gradient encoding was applied in 33 directions using a diffusion-weighted factor, b = 750 s/mm2. The DTI time was approximately 8 min.
Fiber tracking
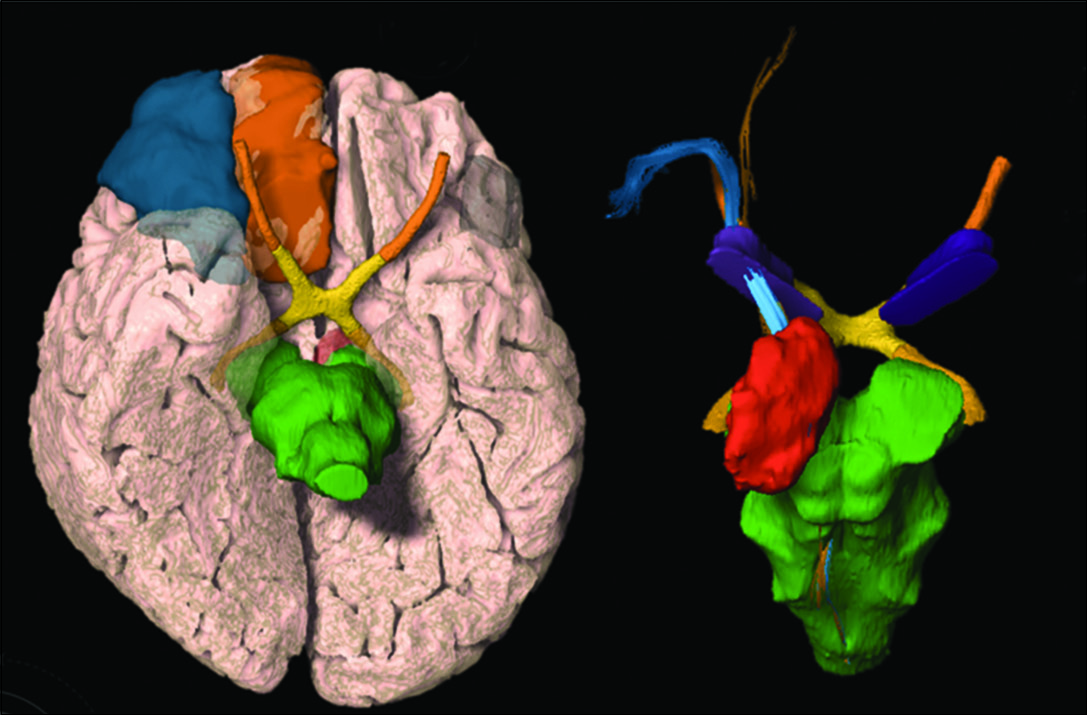
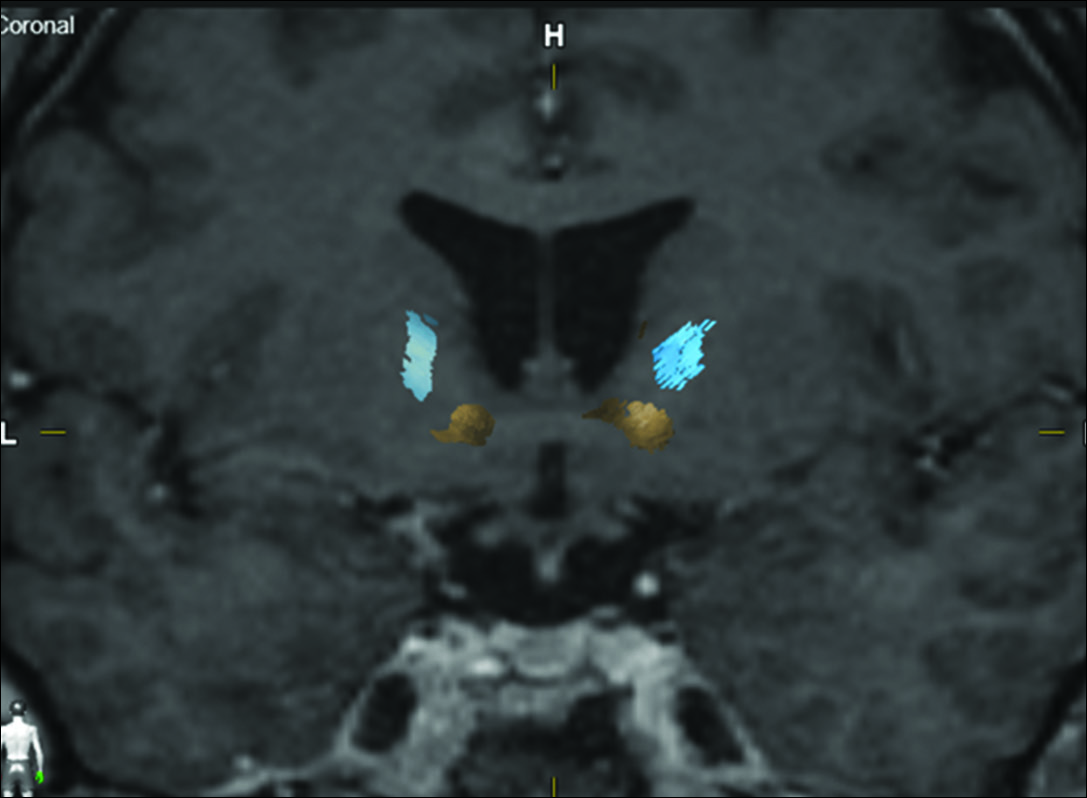
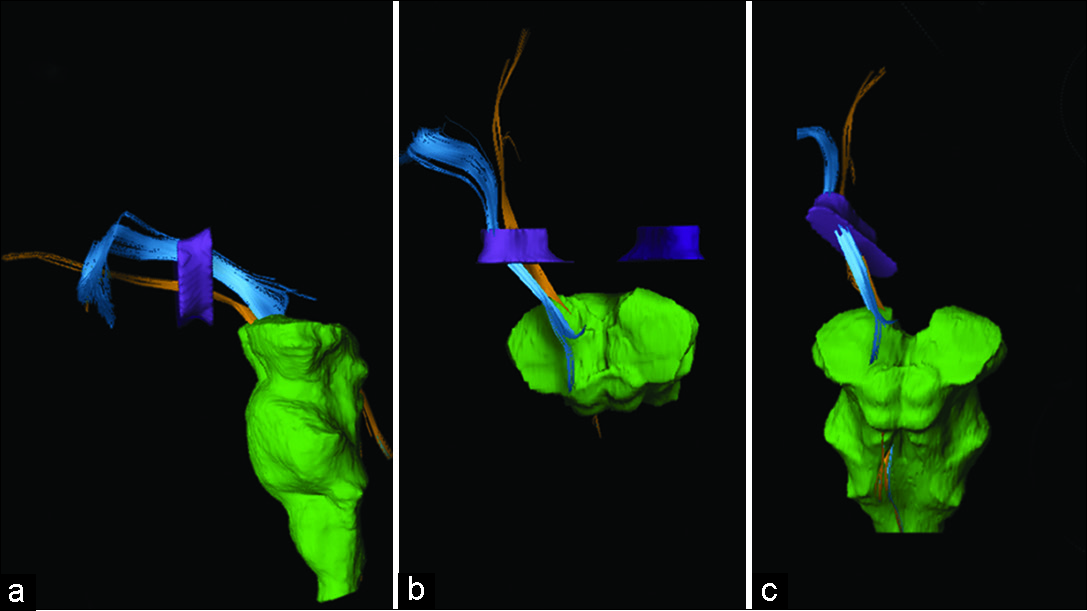
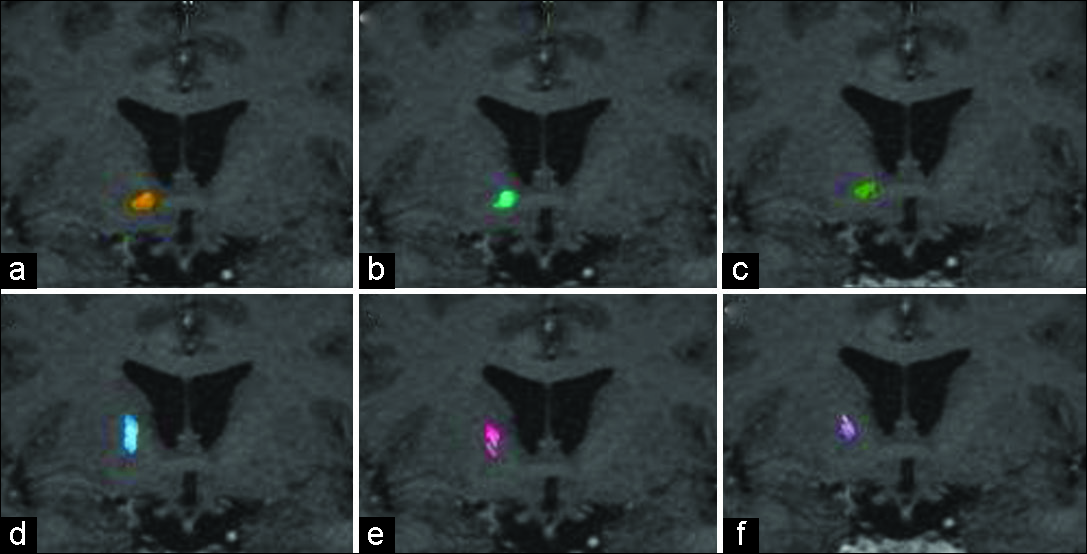
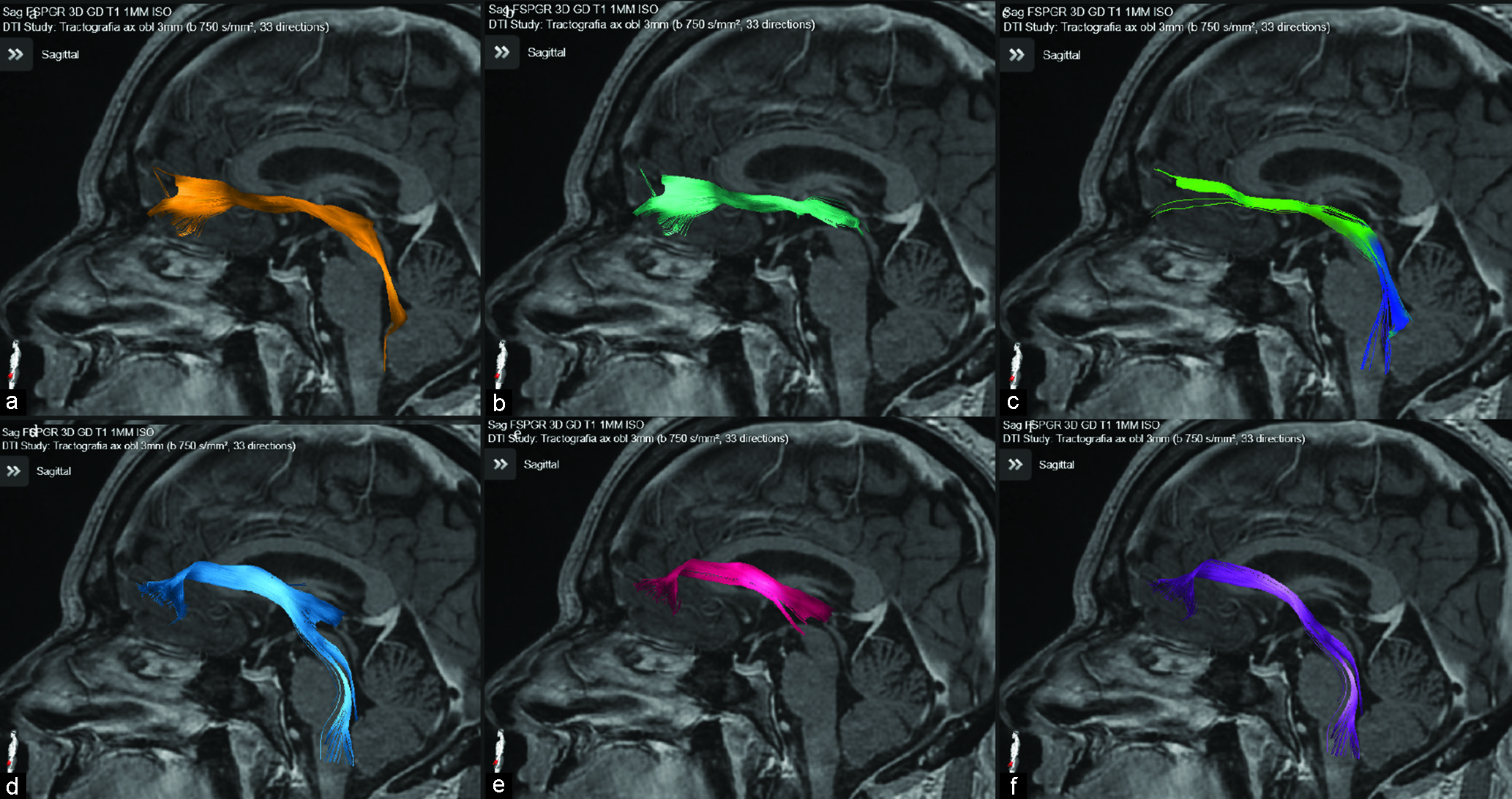
Tractography was reconstructed on a Windows workstation using Brainlab Elements software for cranial surgery planning (Brainlab AG, Munich, Germany). Deterministic fiber tracking (fractional anisotropy [FA] = 0.15, minimum fiber length = 50 mm, and maximal angulation = 13) was used in all cases to reconstruct fibers from OFC. Regions of interest were delineated: medial OFC (straight gyrus and medial orbital gyrus), lateral OFC (anterior, posterior, and lateral orbital gyrus), anterior internal capsule (delineated in coronal view, starting at anterior commissure plane until the plane 10 mm anterior), and brainstem [
Gamma ventral capsulotomy
Radiosurgery was indicated for five patients by psychiatrists who closely evaluated the patients for indication and during follow-up. All procedures were approved by the state of Sao Paulo Regional Medical Board. All patients had Yale–Brown Obsessive Compulsive Scale (Y-BOCS) score >26. They were treated in the gamma unit of the HCor Neuroscience, a Gamma Knife Perfexion (Elekta AB) equipment, between 2014 and 2016. Targets were located at the most ventral borders of the ALIC, touching the shell of the nucleus accumbens. A maximum dose point of 150 Gy and 4 mm collimators was used. The most ventral isocenter was planned 8–10 mm anterior to the posterior border of the anterior commissure. Radiation sensitive structures were protected; mostly, the dose to the optic structures was checked to be lower than 8 Gy. Two OCD patients were reoperated >18 months after the initial bilateral single-shot radiosurgeries and received one additional dorsal shot in each hemisphere. The other three patients were treated with only one ventral shot.
Statistical analyses
Quantitative variables were characterized as mean ± SD. Pathology groups were compared based on fiber tracts volumes, mean FA and mean fiber length. One-way analysis of variance was used, and if a statistically significant difference was detected, Tukey post hoc test for individual comparisons was performed. For comparison of fiber tract statistics between cerebral hemispheres, paired t-test was used. P < 0.05 (two- sided) was considered statistically significant and SPSS (IBM SPSS Statistics, Version 21, IBM, Armonk, NY, USA) was used for all calculations.
RESULTS
Nine PD, six morbid obesity, and five OCD patients were included with a mean (SD) age of 65.4 ± 9.1, 41.0 ± 8.2, and 31.2 ± 5.5, respectively, which are statistically different from each other (P < 0.001). Fourteen patients (70%) were men. A total of 40 cerebral hemispheres were analyzed.
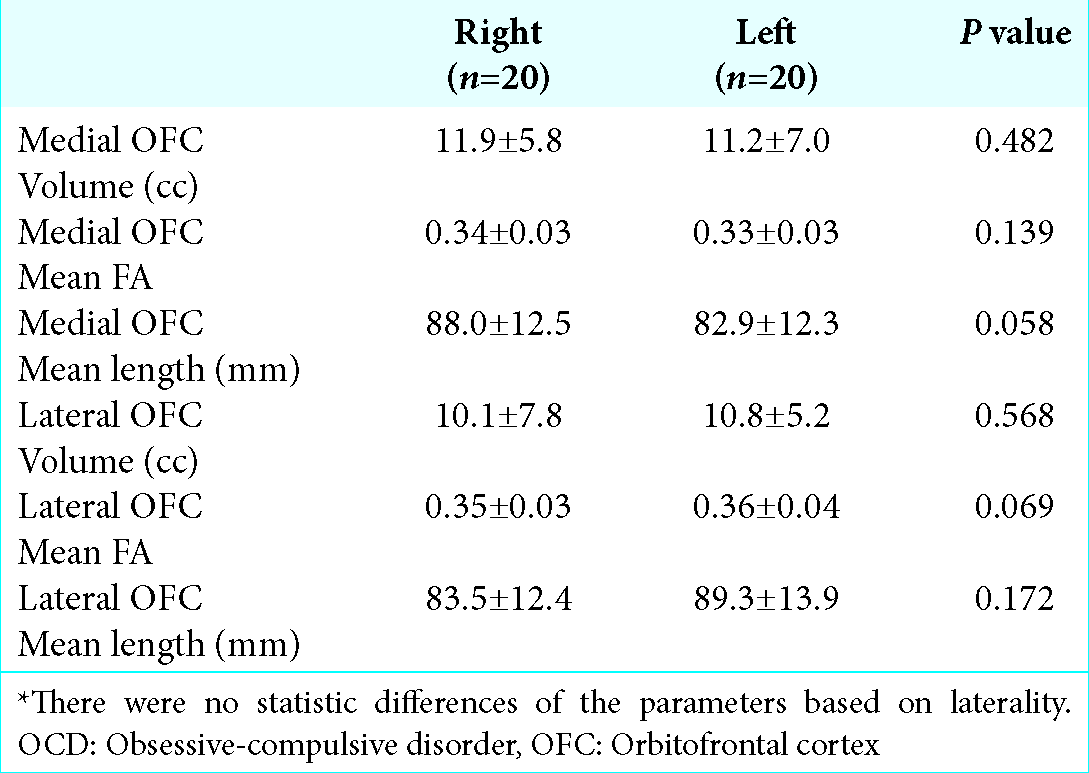
Medial OFC fibers are located more inferior in the ALIC than the lateral OFC fibers in all hemispheres [
The OFC-thalamic component of both lateral and medial OFC fibers is localized more medial than the OFC-brainstem component [
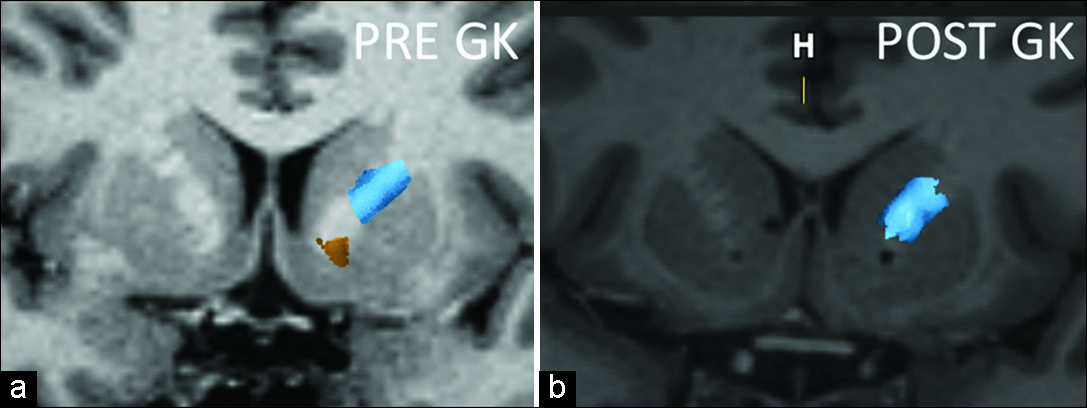
Figure 6:
Coronal magnetic resonance imaging of one of the patients pre- and postradiosurgery. Notice the bilateral inferior location of the gamma capsulotomy and the disappearance of the most inferior fibers (yellow), representing the fibers coming exclusively from the medial left orbitofrontal cortex, side that the tractography is represented. Post GK image shows the sparing of the lateral frontal cortex fibers (blue). The internal capsule lesions were done each with 150 Gy absolute dose and a 4-mm diameter delivery.
Both medial and lateral OFC fiber tracts of PD and morbid obesity patients have lower volume than, respectively, medial and lateral counterparts of OCD patients (P < 0.001). In terms of mean FA and mean fiber length, only PD patients’ medial OFC fibers differ from the other groups [
DISCUSSION
Although GVC for OCD treatment has been used for years by many centers around the world, lesions were based only on anatomical parameters and animal studies,[
Functional imaging studies in OCD are already used and can support the dysfunction of fronto-orbito-striato-thalamo-cortical circuitry by demonstration of hypermetabolic changes in the orbital frontal cortex, thalamus, caudate nucleus, and cingulate region. Changes in these patterns of metabolism distribution in relation to the fibers severed by the Gamma Capsulotomy may further enhance the insights on the region of the ventral anterior internal capsule that must be targeted for OCD treatment.[
Results of GVC for OCD are already described in literature.[
Although anterior capsulotomy is considered an effective and safe procedure, adverse events were already reported by many authors, mainly in the pioneer reports: apathy, disinhibition, problems in executive functioning, attempted suicide, weight gain, urinary incontinence, and severe sexual dysfunctions.[
It is well accepted that the OFC-thalamic fiber tracts play an important role in OCD physiopathology based on functional imaging studies as well as on the historical results of gamma and radiofrequency capsulotomy.[
A double isocenter lesion has been proposed.[
There are important limitations in this study. Although the ROI volumes were delineated based on strict anatomical landmarks by a single observer (BFOS), it is possible interobserver variability. In addition, it must be considered the reliability of DTI tractography knowing the possibility of interalgorithm variability.[
In addition, this study shows that there are differences between the volumes of the tracts studied in patients with different pathologies. Morbid obesity, PD, and OCD patients seem to have different volumes of tracts; interestingly, the patients with OCD have a higher volume of tracts, suggesting a hyperactivity of these pathways, as noted in other studies. This justifies additional studies trying to identify specific targets based on DTI tractography for OCD patients and possibly for other pathologies. However, it is important to highlight that, for better conclusions, it is necessary controlling for age and brain volume. For example, PD patients had the smallest volume of fibers passing through the ALIC, likely in agreement with executional task impairment due to compromised frontal lobe function commonly observed in neuropsychological tests performed for surgery eligibility evaluation, although simple age differences among the three groups cannot be discarded as the reason for this finding.
The DTI tractography is being progressively implemented in neurosurgery.[
Studies should also compare DTI tractography of healthy controls with those of OCD patients. Further analysis relying on OFC fiber bundles distribution in the ALIC may permit refinement of neurosurgical targeting in OCD patients. Future investigations should correlate the localization of neurosurgical procedures with patient outcomes using strategies such as the ones of this paper.
CONCLUSIONS
Medial and lateral OFC tract fibers have a general standard distribution in the ALIC. Fibers originating in the lateral orbitofrontal are located dorsally to the ones coming from the medial OFC at the internal capsule. There is a level of intersection, and exact topography of fiber bundles is variable among individuals. These limitations of discerning these bundles may improve with the development of the DTI tractography technique.
References
1. Barkhoudarian G, Klochkov T, Sedrak M, Frew A, Gorgulho A, Behnke E. A role of diffusion tensor imaging in movement disorder surgery. Acta Neurochir (Wien). 2010. 152: 2089-95
2. Cottraux J, Bouvard MA, Milliery M. Combining pharmacotherapy with cognitive-behavioral interventions for obsessive-compulsive disorder. Cogn Behav Ther. 2005. 34: 185-92
3. Gomes JG, Gorgulho AA, de Oliveira Lopez A, Saraiva CW, Damiani LP, Passaro AM. The role of diffusion tensor imaging tractography for Gamma Knife thalamotomy planning. J Neurosurg. 2016. 125: 129-38
4. Gouvea F, Lopes A, Greenberg B, Canteras M, Taub A, Mathis M. Response to sham and active gamma ventral capsulotomy in otherwise intractable obsessive-compulsive disorder. Stereotact Funct Neurosurg. 2010. 88: 177-82
5. Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: Stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010. 35: 317-36
6. Kondziolka D, Flickinger JC, Hudak R. Results following gamma knife radiosurgical anterior capsulotomies for obsessive compulsive disorder. Neurosurgery. 2011. 68: 28-32
7. Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: Implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011. 31: 10392-402
8. Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: Relevance of the right hemisphere. Neurosurgery. 1999. 44: 452-8
9. Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: The search for a valid target. Neurosurgery. 2007. 61: 1-11
10. Lopes AC, Greenberg BD, Canteras MM, Batistuzzo MC, Hoexter MQ, Gentil AF. Gamma ventral capsulotomy for obsessive-compulsive disorder: A randomized clinical trial. JAMA Psychiatry. 2014. 71: 1066-76
11. Lopes AC, Greenberg BD, Noren G, Canteras MM, Busatto GF, de Mathis ME. Treatment of resistant obsessive-compulsive disorder with ventral capsular/ventral striatal gamma capsulotomy: A pilot prospective study. J Neuropsychiatry Clin Neurosci. 2009. 21: 381-92
12. Makris N, Rathi Y, Mouradian P, Bonmassar G, Papadimitriou G, Ing WI. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): Precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive disorder (OCD). Brain Imaging Behav. 2016. 10: 1054-67
13. Pepper J, Hariz M, Zrinzo L. Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: A review of the literature. J Neurosurg. 2015. 122: 1028-37
14. Pujol S, Wells W, Pierpaoli C, Brun C, Gee J, Cheng G. The DTI challenge: Toward standardized evaluation of diffusion tensor imaging tractography for neurosurgery. J Neuroimaging. 2015. 25: 875-82
15. Rück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K. Capsulotomy for obsessive-compulsive disorder: Long-term follow-up of 25 patients. Arch Gen Psychiatry. 2008. 65: 914-21
16. Sedrak M, Gorgulho A, De Salles AF, Frew A, Behnke E, Ishida W. The role of modern imaging modalities on deep brain stimulation targeting for mental illness. Acta Neurochir Suppl. 2008. 101: 3-7
17. Sheehan JP, Patterson G, Schlesinger D, Xu Z. Gamma knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder. J Neurosurg. 2013. 119: 1112-8
18. Vos T, Mathers CD. The burden of mental disorders: A comparison of methods between the Australian burden of disease studies and the global burden of disease study. Bull World Health Organ. 2000. 78: 427-38
19. Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004. 132: 69-79