- College of Medicine, University of Baghdad, Baghdad, Iraq,
- Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
- Department of Neurosurgery, Johns Hopkins University, Baltimore, MD, USA,
- Department of Surgery, College of Medicine, University of Al-Qadisiyah, Diwaniyah, Iraq.
Correspondence Address:
Zahraa A. Alsubaihawi1
Department of Neurosurgery, Neurosurgery Teaching Hospital, Baghdad, Iraq,
DOI:10.25259/SNI_238_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aktham O. Al-Khafaji1, Zahraa F. Al-Sharshahi2, Ryan P. Lee3, Zahraa A. Alsubaihawi1, Ali A. Dolachee4, Samer S. Hoz2. Unilateral absence of the internal carotid artery associated with anterior communicating artery aneurysms: Systematic review and a proposed management algorithm. 01-Aug-2020;11:221
How to cite this URL: Aktham O. Al-Khafaji1, Zahraa F. Al-Sharshahi2, Ryan P. Lee3, Zahraa A. Alsubaihawi1, Ali A. Dolachee4, Samer S. Hoz2. Unilateral absence of the internal carotid artery associated with anterior communicating artery aneurysms: Systematic review and a proposed management algorithm. 01-Aug-2020;11:221. Available from: https://surgicalneurologyint.com/surgicalint-articles/10175/
Abstract
Background: Absence or hypoplasia of the internal carotid artery (ICA) is a rare congenital anomaly that is mostly unilateral and highly associated with other intracranial vascular anomalies, of which saccular aneurysm is the most common. Blood flow to the circulation of the affected side is maintained by collateral pathways, some of which include the anterior communicating artery (Acom) as part of their anatomy. Therefore, temporary clipping during microsurgery on Acom aneurysms in patients with unilateral ICA anomalies could jeopardize these collaterals and place the patient at risk of ischemic damage. In this paper, we review the literature on cases with a unilaterally absent ICA associated with Acom aneurysms and provide an illustrative case.
Methods: We combined our experience of one case of a unilaterally absent ICA associated with an Acom aneurysm with the 33 existing publications on the same subject in the literature, for a total of 40 cases. We provide a detailed systematic literature review of this association of vascular anomalies, exploring different aspects regarding the collateral pathways and how they impact management strategies and propose a management algorithm to deal with such association.
Results: The mean age was 48.2 ± 16.5 years. The aneurysmal rupture was the most common presentation (75%). Agenesis was observed in 70% of patients, followed by hypoplasia (20%) and, finally, aplasia (10%). Lie Type A was the most common pattern of collaterals (50%), with Types B and D being of almost equal proportions. Most aneurysms were located at the A1-Acom junction contralateral to the anomalous side (Fisher’s Exact test; P = 0.03). One case of temporary clipping was reported in the literature.
Conclusion: Acom aneurysms in patients with unilateral ICA anomalies, given they are more commonly present contralaterally, could be of acquired etiology, warranting periodic screening in asymptomatic patients. Temporary clipping might be safe in patients with Type D collateral pattern, while those with Types A or B may require intraoperative rupture risk assessment and a tailored management plan to avoid disrupting collateral flow and causing ischemia.
Keywords: Absence, Aneurysm, Anterior communicating artery, Hypoplasia, Internal carotid artery, Unilateral
INTRODUCTION
Congenital anomalies of the internal carotid artery (ICA) is an umbrella term encompassing three developmentally distinct, although interchangeably termed, conditions affecting the ICA, namely, agenesis, aplasia, and hypoplasia.[
Agenesis refers to the complete absence of an artery or its primordium, while aplasia is used to describe an undeveloped ICA with the presence of some of its primordia, such as remnant vessel segments, or a sign of its presence such as a patent ipsilateral carotid canal. These two terms are often collectively referred to in the literature as congenital absence of the ICA.[
These anomalies rarely cause symptoms when present in isolation.[
The anterior communicating artery (Acom) is reported to be the most common location for intracranial aneurysms associated with congenital ICA anomalies.[
In this article, we provide a detailed, systematic review of the available literature on the unilateral absence of the ICA associated with Acom aneurysms. Clinical aspects of the available cases are abstracted, statistically analyzed and discussed in regards to their etiology, the possible collateral pathways, and their impact on treatment options. The available data are also used to propose a management algorithm for dealing with such conditions.
Illustrative case
A 51-year-old man with a history of hypertension was referred to the Neurosurgical Teaching Hospital in Baghdad after complaints of sudden, severe headache associated with nausea and forceful vomiting. Physical examination showed intact consciousness and normal vital signs. Pupillary examination showed bilaterally equal and reactive pupils of normal size (about 3 mm) and round shape. Cranial nerves examination was unremarkable. The patient had severe neck stiffness and left hemiparesis (muscle power was 3/5).
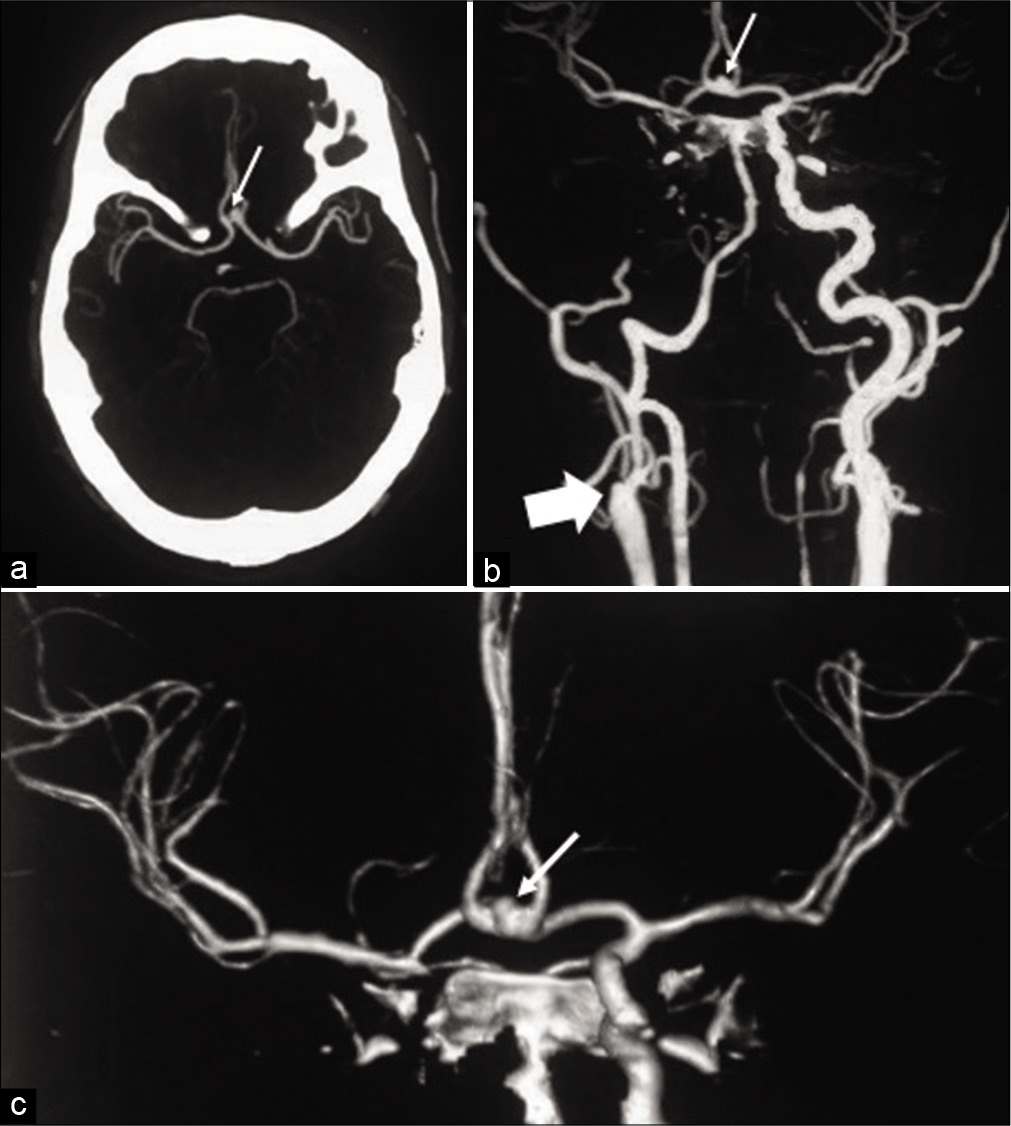
Non-contrast computed tomography (CT) scan revealed diffuse thick subarachnoid hemorrhage (SAH) with mild ventricular dilatation. CT angiogram (CTA) shown in [
Figure 1:
Computed tomography angiogram (CTA) (a) and 3D-reconstructed CTA (b and c) showing a left A1-ACoA junction aneurysm with right superior-anterior projection (thin arrows). The right internal carotid artery (ICA) is not visualized down to its origin from the common carotid artery (thick arrow). The right anterior cerebral artery and middle cerebral artery are supplied by the left ICA through the left A1-ACoA pathway.
Microsurgical treatment was performed through a left pterional trans-sylvian approach. During surgery, absence of the right ICA was visually confirmed, and the left A1-Acom complex showed vasospasm. In addition, the aneurysm neck was very wide and its manipulation was difficult without temporary clipping of the left A1, a procedure that would risk blood flow to the right ACA and MCA. Intraoperatively, the aneurysm showed a punctate source of bleeding. A low- voltage bipolar cautery, set at 5–7, was used to shrink the bleeding source. Once bleeding has been controlled, the aneurysm was secured with muscle wrapping. Using low- voltage bipolar cautery to repair vascular injuries or to shrink punctate bleeding is a common practice in cerebrovascular surgery. After surgery, the patient’s muscle power improved, he had no further complications, and was discharged on day 7 postoperatively. The lesion remained stable on follow-up imaging.
LITERATURE REVIEW
Methods
A systematic online literature search was performed on the April 14, 2020, for reports on unilateral congenital ICA anomalies associated with Acom aneurysms. PubMed (Medline 1966–2020) and Google Scholar were used for the review, and the search algorithm was as follows: “(((Acom) OR (ACoA) OR (AComA) OR (anterior communicating artery)) AND ((aneurysm) OR (intracranial aneurysm [MeSH])) AND (unilateral) AND (((ica) OR (internal carotid artery) OR (carotid artery, internal [MeSH])) AND ((anomaly) OR (absence) OR (aplasia) OR (agenesis) OR (hypoplasia)))).” An additional filter was applied for articles published on humans. The search returned 526 peer- reviewed manuscripts. A systematic abstract screening was performed, and only articles that reported on unilateral congenital ICA anomalies with associated Acom aneurysms were included for final analysis. In addition, the reference list of each article was screened for additional articles. All non- English literature was translated.
The gender, age, presenting signs/symptoms, type, side of the ICA anomaly, and Lie classification for collateral pattern were recorded for each case. Concerning the Acom aneurysm, the side (of its junction with A1), whether it was ruptured or not at presentation, course of treatment, and outcome was recorded. Statistical work was done using IBM Statistical Package for the Social Sciences version 26. Continuous variables were tested for the difference using independent samples t-test. Categorical variables were tested for correlation using Fisher’s Exact test. The significance level was set at P < 0.005.
RESULTS
Our review yielded a total of 33 articles reporting 39 cases of unilateral ICA anomalies associated with Acom aneurysms, aside from the case we present in this article.[
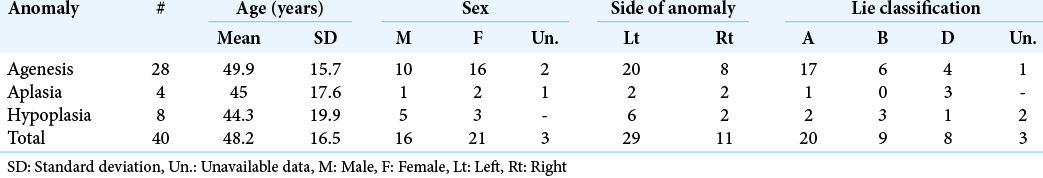
The mean age of patients at presentation was 48.2 ± 16.5 years. Twenty-one (52.5%) of the patients were females, and 29 (72.5%) of cases had a left-sided anomaly of the ICA. Of the aforementioned anomalies, agenesis was most common (70%), followed by hypoplasia (20%). There was no significant difference in age at presentation between males and females (P = 0.84) or the types of anomalies (P = 0.67). Half of all cases had a Lie Type A collateral pattern, with the remaining half having either Types B or D patterns in almost equal proportions (22.5% and 20%, respectively).
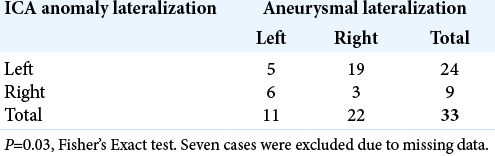
Thirty (75%) of the patients presented with signs of SAH; namely, sudden severe headache with or without nausea and vomiting (three of which were due to ruptured aneurysms other than the Acom aneurysm), while the remaining cases had different presentations (three chronic headache, two incidental, one dysarthria, one seizures and microcephaly, one progressive hearing loss, one numbness, and one unavailable data). Additional vascular anomalies were reported in nine patients; those included absence of the CCA, ECA, and vertebral artery, “cross-over” duplication of the MCA, moyamoya phenomena, and arachnoid cyst. Surgically treated patients had a significantly better outcome compared to non-operative treated patients (Fisher’s Exact test, P = 0.004).
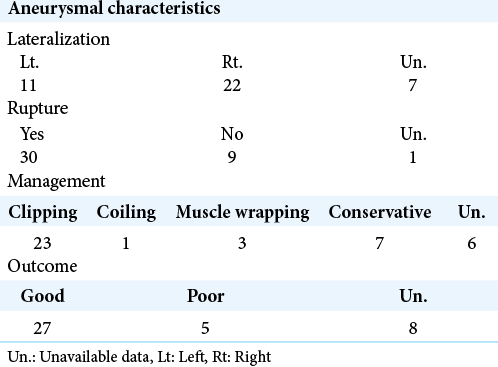
The demographic data of the study group, as well as characteristics of the Acom aneurysms, are summarized in [
DISCUSSION
Given the low prevalence of congential ICA anomalies, the available literature is mainly related to their epidimiology. There has not been much discussion on details of management and follow-up for these patients, particularly for the special circumstance of association with Acom aneurysms. Hence, our review suffers from an inherent lack of clinical data on surgical and long-term follow-up details, and we are therefore forced to depend on anecdotal evidence and single incidental reports from the literature in providing recommendations for the management of such patients. In our case, the bleeding point was cauterized, and the aneurysm was muscle wrapped. This management strategy was also adopted by Burmester and Stender. However, other management options, including clipping, and endovascular coiling, should all be considered by the surgeon when faced with such lesions.
Patterns of collateral flow in unilateral absence of the ICA
In his original 1968 thesis, Lie describes six patterns of collateralization to supply blood to the hemisphere affected by ICA agenesis, aplasia, or severe hypoplasia.[
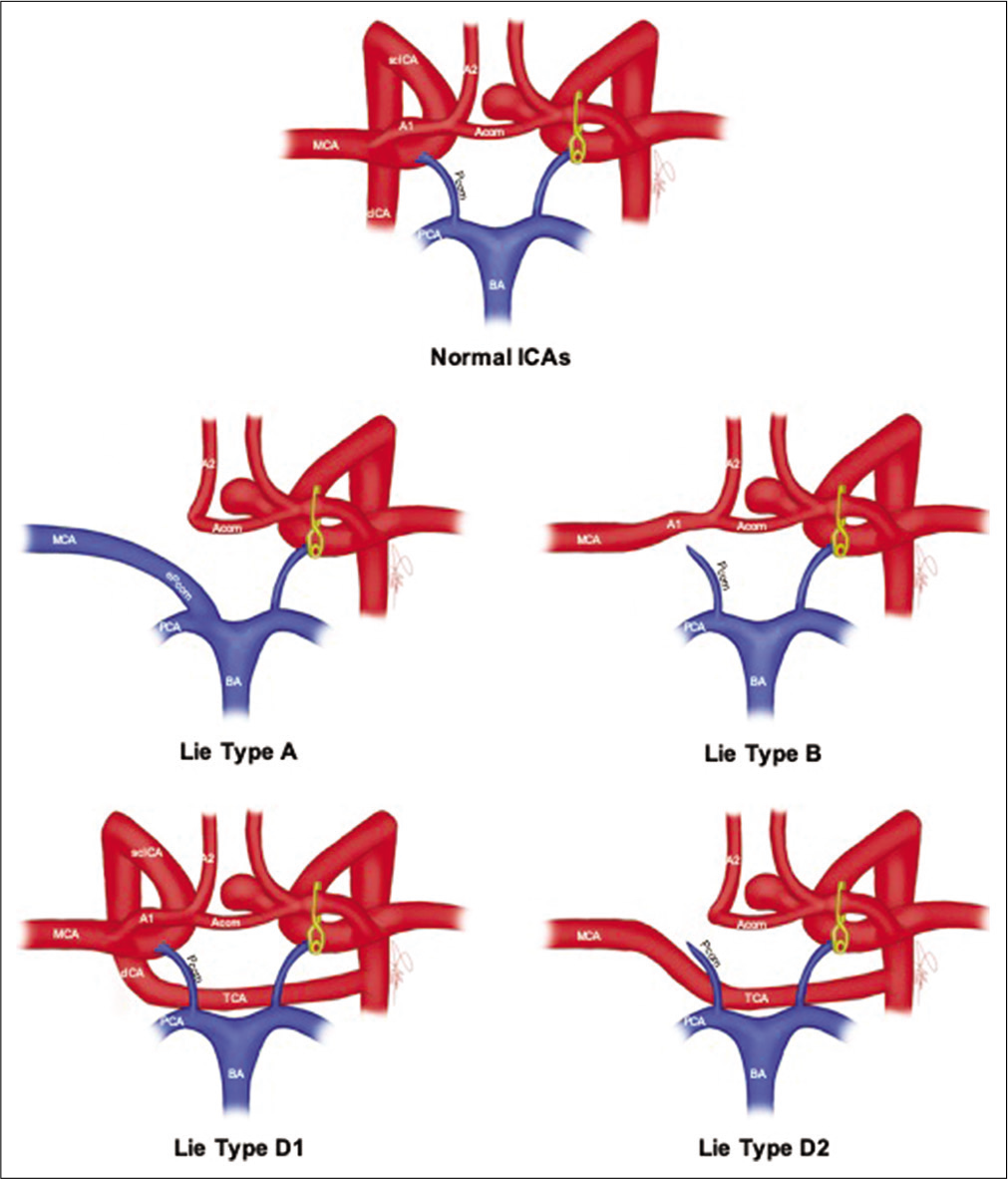
In Type A, the ACA of the affected side is supplied by the contralateral ICA through the contralateral A1 and Acom, while the MCA of the affected side is supplied by the posterior circulation through an enlarged ipsilateral Pcom. In type B, both the ACA and MCA of the affected side are supplied by the contralateral ICA through the contralateral A1 and Acom. Type D describes a “trans-cavernous” pattern of collateralization, in which an embryonic remnant vessel originating from the cavernous portion of the normal ICA persists. This remnant vessel reconstitutes blood flow either by anastomosing with the cavernous portion of the affected ICA (a pattern we refer to as D1), or by directly supplying the MCA of the affected side (D2).
Of particular interest, we observed that patients with Lie Type D collateral pattern, the rarest type in unilateral anomalies, displayed a much higher affinity for Acom aneurysms compared to other types. Of the total 20 cases of ICA anomaly with type D pattern reported in the literature, eight of them had saccular intracranial aneurysms, and all of them had an Acom aneurysm (sole in six cases, associated with other aneurysms in one case).[
Etiology of Acom aneurysms associated with unilateral absence or hypoplasia of ICA
Although the exact etiological factors causing intracranial saccular aneurysms in patients with anomalies of the ICA are not well studied, several articles propose two main possible mechanisms. The congenital theory implies the coexistence of the aneurysm alongside the ICA anomaly since fetal life.[
In contrast, the acquired theory implicates the changes in blood flow brought about by the abnormal flow vectors within the collateral pathways as a cause for the development of the aneurysms.[
Need for periodic aneurysm screening
Despite the rarity of ICA absence or hypoplasia, which are estimated to be prevalent in <0.01% of the population, the prevalence of intracranial saccular aneurysms in association with them (ranging from 25 to 67%) is much higher compared to that of the normal general population (2–4%).[
Magnetic resonance angiography (MRA) might be preferable in this setting over catheter or CT angiography, given its non-invasive, non-ionizing properties, and good sensitivity profile. The previous studies support the suggestion that MRA is a valid screening tool for intracranial aneurysms, as recent advances have significantly improved its sensitivity and specificity.[
Preservation of collateral flow during microneurosurgery
Temporary clipping is often employed during microvascular clipping of intracranial aneurysms, either in an elective manner to acquire proximal or distal control of vessels to facilitate the manipulation of the aneurysm complex, or as a rescue measure to control bleeding caused by intraoperative rupture (IOR) of the aneurysm. For Acom aneurysms, the temporary clipping usually involves the A1 segment of the ipsilateral ACA. Temporary clipping is not associated with additional risk of delayed cerebral infarction or delayed ischemic neurological deficits in patients with bilaterally normal ICA formation.[
Figure 2:
Figure artistic depiction of the anatomical position of ACOM aneurysm in normal and unilateral congenitally absent ICAs and its relation to collateral blood flow patterns. CICA: Cavernous internal carotid artery, SCICA: Supraclinoid internal carotid artery, A1, A2: Segments 1 and 2 of the anterior cerebral artery, ACOM: Anterior communicating artery, MCA: Middle cerebral artery, BA: Basilar artery, PCA: Posterior cerebral artery, PCOM: Posterior communicating artery, EPCOM: Enlarged posterior communicating artery, TCA: Transcavernous anastomosis.
For Type D1, blood flow is reinstituted to the affected ICA at its cavernous portion far from the Acom, and as such, no special considerations need to be taken when managing such a combination. On the other hand, the contralateral A1 segment and Acom constitute a major collateralization pathway in ICA anomaly patients with Type A, B, and D2 patterns.
In Type A, the collateral pathway supplies the ACA, while the MCA is supplied by the posterior circulation through an enlarged ipsilateral Pcom. Disruption of the A1-Acom pathway by temporary clipping in such patients would compromise blood flow to the affected side ACA, sparing the MCA. Bhaskar et al.[
In contrast, Type B, in which the collateral pathway supplies blood flow to both the ipsilateral ACA and MCA, the anatomy implies that disruption of these collaterals would cutoff all blood supply to a major portion of the affected hemisphere. Based on the above flow patterns for Types A, B, and D2, it is a reasonable practice to assess the risk of IOR before intervention and determine the course of action accordingly.
Endovascular intervention is a very viable initial treatment option for intracranial aneurysms, both ruptured and unruptured.[
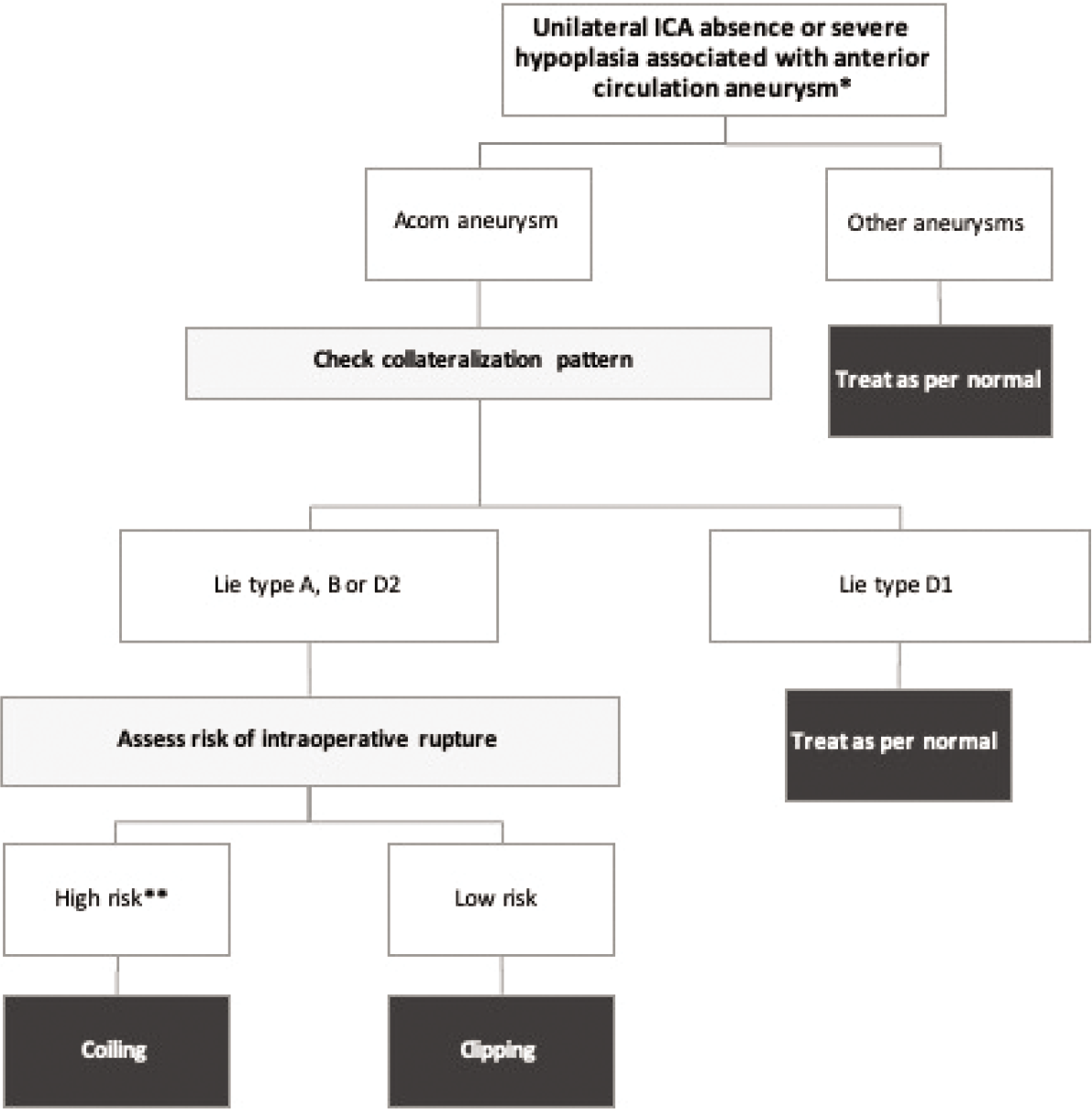
Based on all data discussed above, we have devised a simple algorithm for the management of patients presenting with unilateral absence or severe hypoplasia of the ICA in the context of associated aneurysms, as shown in
Figure 3:
Proposed outline for anterior circulation aneurysm-contextual management of patients presenting with unilateral congenital ICA anomalies. *For ICA anomalies with no associated aneurysms, we propose periodic screening, preferably using MRA as a non-invasive safe method. **Generally, ruptured aneurysms have a higher risk of IOR compared to unruptured aneurysms. High risk group (unruptured aneurysms): large (for clipping) or very small (for coiling) size, anteriorly directed dome, irregular shape with daughter cysts, high aspect ratio. High risk group (ruptured aneurysms): High modified Fisher’s or Hunt and Hess grade, rebleeding before intervention.
Some studies have suggested that ruptured Acom aneurysms have the greatest risk for re-rupture during microsurgery among all aneurysmal sites.[
for assessing IOR risk in ruptured aneurysms include high modified Fisher’s or Hunt and Hess grades and rebleeding before intervention.[
CONCLUSION
Intracranial saccular aneurysms are a common finding in patients with unilateral congenital anomalies of the ICA and could be of acquired etiology in such patients. Periodic screening for patients with these congenital anomalies for the development of aneurysms could be warranted. Acom aneurysms in association with Lie Type D1 collateral pattern can be managed as in patients with bilaterally normal ICAs. Acom aneurysms associated with Lie Types A, B, or D2 collateral patterns might require special considerations during microsurgical clipping, as the application of temporary clips could compromise collateral blood flow and cause ischemic damage. Endovascular therapy should be more strongly considered in those cases.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Afifi AK, Godersky JC, Menezes A, Smoker WR, Bell WE, Jacoby CG. Cerebral hemiatrophy, hypoplasia of internal carotid artery, and intracranial aneurysm: A rare association occurring in an infant. Arch Neurol. 1987. 44: 232-5
2. Armand JP, Dousset V, Viaud B, Huot P, Chehab Z, Dos ES. Agenesis of the internal carotid artery associated with an aneurysm of the anterior communicating artery. J Neuroradiol. 1996. 23: 164-7
3. Baek GS, Koh EJ, Lee WJ, Choi HY. Congenital hypoplasia of internal carotid artery accompanying with cerebral aneurys. J Korean Neurosurg Soc. 2007. 41: 343-6
4. Bernini FP, Cioffi FA, Muras I, Rinaldi F, Vaino R. Agenesis of the right internal carotid artery associated with an aneurysm of the anterior communicating artery. Case report. Acta Neurochir (Wien). 1980. 54: 257-63
5. Bhaskar S, Singh S, Shivender S, Singh A. Agenesis of internal carotid artery with anterior communicating artery aneurysm. Neurol India. 2012. 60: 547
6. Brown RD, Huston J, Hornung R, Foroud T, Kallmes DF, Kleindorfer D. Screening for brain aneurysm in the familial intracranial aneurysm study: Frequency and predictors of lesion detection. J Neurosurg. 2008. 108: 1132-8
7. Burmester K, Stender A. 2 cases of unilateral aplasia of the internal carotid artery in simulataneous aneurysm formation in the anterior area of the circle of Willis (On the problem of the combination of saccular aneurysms of the cerebral arteries with other abnormalities). Acta Neurochi (Wien). 1961. 9: 367-78
8. Chen J, Raden M, Lin C. Congenital absence of the internal carotid artery with intercavernous anastomosis. Radiol Case Rep. 2019. 14: 1021-6
9. Chen L, Liu JM, Zhou D. Congenital absence of the right common carotid artery, internal carotid artery and external carotid artery associated with anterior communicating artery aneurysm: A rare case. Neurol Sci. 2008. 29: 485-7
10. Chowdhury T, Cappellani RB, Sandu N, Schaller B, Daya J. Perioperative variables contributing to the rupture of intracranial aneurysm: An update. Sci World J. 2013. 2013: 396404
11. Clarós P, Bandos R, Gilea I, Clarós A, Capdevila A, Rodríguez JG. Major congenital anomalies of the internal carotid artery: Agenesis, aplasia and hypoplasia. Int J Pediatr Otorhinolaryngol. 1999. 49: 69-76
12. Cohen MM, Kristiansen K. Association of aneurysm with anomalies of the arteries at the base of the brain. Zentralbl Gesamte Neurol Psychiatry. 1957. 143: 11
13. Czarnecki EJ, Silbergleit R, Mehta BA, Sanders WP. Absence of the supraclinoid internal carotid artery in association with intracranial aneurysms. Neuroradiology. 1998. 40: 11-4
14. Demirgil B, Tuğcu B, Günaldi Ö, Postalcı L, Günal M, Tanrıverdi O. Agenesis of the left internal carotid artery associated with right anterior cerebral artery A1 segment bifurcation aneurysm: A case report. Minim Invasive Neurosurg. 2007. 50: 300-3
15. Elijovich L, Higashida RT, Lawton MT, Duckwiler G, Giannotta S, Johnston SC. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: The carat study. Stroke. 2008. 39: 1501-6
16. Gibbs GF, Huston J, Bernstein MA, Riederer SJ, Brown RD. Improved image quality of intracranial aneurysms: 3.0-T versus 1.5-T time-of-flight MR angiography. Am J Neuroradiol. 2004. 25: 84-7
17. Goertz L, Hamisch C, Telentschak S, Kabbasch C, von Spreckelsen N, Stavrinou P. Impact of aneurysm shape on intraoperative rupture during clipping of ruptured intracranial aneurysms. World Neurosurg. 2018. 118: e806-12
18. Horie N, Tsutsumi K, Kaminogo M, Morikawa M, Kitagawa N, Nagata I. Agenesis of the internal carotid artery with transcavernous anastomosis presenting with an anterior communicating artery aneurysm-a case report and review of the literature. Clin Neurol Neurosurg. 2008. 110: 622-6
19. Huber G. Intracranial carotid anastomosis and partial aplasia of an internal carotid artery. Neuroradiology. 1980. 20: 207-12
20. Ishihara H, Ishihara S, Niimi J, Neki H, Kakehi Y, Uemiya N. Risk factors and prevention of guiding catheter-induced vasospasm in neuroendovascular treatment. Neurol Med Chir (Tokyo). 2015. 53: 261-5
21. Itokawa H, Moriya M, Fujimoto M, Kato A, Okamoto N, Noda M. A giant anterior communicating artery aneurysm associated with hypoplasia of the unilateral internal carotid artery. J Neuroendovasc Ther. 2009. 3: 17-23
22. Keedy A. An overview of intracranial aneurysms. Mcgill J Med. 2006. 9: 141-6
23. Kim SM, Kim SH, Seo DW, Lee KW. Intraoperative neurophysiologic monitoring: Basic principles and recent update. J Korean Med Sci. 2013. 28: 1261-9
24. Kumagai K, Takeuchi S, Otani N, Komiyama M, Mori K. Agenesis of the internal carotid artery with transcavernous anastomosis associated with anterior communicating artery aneurysms. Asian J Neurosurg. 2017. 12: 801-3
25. Kunishio K, Yamamoto Y, Sunami N, Asari S. Agenesis of the left internal carotid artery, common carotid artery, and main trunk of the external carotid artery associated with multiple cerebral aneurysms. Surg Neurol. 1987. 27: 177-81
26. Lagarde C, Vigouroux R, Perrouty P. Terminal agenesis of internal carotid, aneurysm of anterior communicating; radiologic documents. Radiol Electrol Arch Electr Medicale. 1957. 38: 939-41
27. Lee JH, Oh CW, Lee SH, Han DH. Aplasia of the internal carotid artery. Acta Neurochir (Wien). 2003. 145: 117-25
28. Lhermitte F. Hypoplasia of the internal carotid artery. Neurology. 1968. 18: 439-46
29. Li S, Hooda K, Gupta N, Kumar Y. Internal carotid artery agenesis: A case report and review of literature. Neuroradiol J. 2017. 30: 186-91
30. Lie T.editors. Congenital Anomalies of the Carotid Arteries. Including the Carotid-Basilar and Carotid-Vertebral Anastomoses. An Angiographic Study and a Review of the Literature. Amsterdam: Excerpta Medica; 1968. p.
31. Malinova V, Schatlo B, Voit M, Suntheim P, Rohde V, Mielke D. The impact of temporary clipping during aneurysm surgery on the incidence of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017. 129: 84-90
32. Matsukawa H, Uemura A, Fujii M, Kamo M, Takahashi O, Sumiyoshi S. Morphological and clinical risk factors for the rupture of anterior communicating artery aneurysms. J Neurosurg. 2013. 118: 978-83
33. Midkiff RB, Boykin MW, McFarland DR, Bauman JA. Agenesis of the internal carotid artery with intercavernous anastomosis. Am J Neuroradiol. 1995. 16: 1356-9
34. Molyneux A, Kerr R. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. J Stroke Cerebrovasc Dis. 2002. 11: 304-14
35. Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND. Is aspect ratio a reliable predictor of intracranial aneurysm rupture?. Neurosurgery. 2004. 54: 1343-8
36. Naito T, Mikami Y, Nobuoka J, Yoshimoto I, Oshima H. Agenesis of internal carotid artery associated with aneurysm of anterior communicating artery (author’s transl). No Shinkei Geka. 1977. 5: 593-6
37. Nakai H, Kawata Y, Aizawa S, Tanaka T, Yonemasu Y. Unilateral agenesis of the internal carotid artery in a patient with ruptured aneurysm of the anterior communicating artery; a case report. No Shinkei Geka. 1992. 20: 893-8
38. Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell RM. Temporary vessel occlusion for aneurysm surgery: Risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996. 84: 785-91
39. Oppong MD, Pierscianek D, Ahmadipour Y, Dinger TF, Dammann P, Wrede KH. Intraoperative aneurysm rupture during microsurgical clipping: Risk re-evaluation in the post-international subarachnoid aneurysm trial era. World Neurosurg. 2018. 119: e349-56
40. Orakdöğen M, Berkman Z, Erşahin M, Biber N, Somay H. Agenesis of the left internal carotid artery associated with anterior communicating artery aneurysm: Case report. Turk Neurosurg. 2007. 17: 273-6
41. Petrela M, Kurti X, Xhumari A, Leka LI, Anastasi V, Vreto G. Cross-over duplication of middle cerebral artery, agenesis of internal carotid artery and saccular aneurysms. Acta Neurochir (Wien). 1987. 84: 73-6
42. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: Current status. Stroke. 2013. 44: 2046-54
43. Quint DJ, Boulos RS, Spera TD. Congenital absence of the cervical and petrous internal carotid artery with intercavernous anastomosis. Am J Neuroradiol. 1989. 10: 435-9
44. Rinkel GJ. Intracranial aneurysm screening: Indications and advice for practice. Lancet Neurol. 2005. 4: 122-8
45. Ruigrok YM, Buskens E, Rinkel GJ. Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke. 2001. 32: 1173-5
46. Schuette AJ, Hui FK, Spiotta AM, Obuchowski NA, Gupta R, Moskowitz SI. Endovascular therapy of very small aneurysms of the anterior communicating artery: Five-fold increased incidence of rupture. Neurosurgery. 2011. 68: 731-7
47. Shigemori M, Kojo N, Miyagi J, Watanabe M, Kuramoto S. Agenesis of the left internal carotid artery associated with an aneurysm of the anterior communicating artery. Neurol Med Chir (Tokyo). 1980. 20: 73-9
48. Shukla SK, Parashar S, Saxena S. Congenital Absence of unilateral internal carotid artery with an intracerebral aneurysm. Asian J Neurosurg. 2018. 13: 774-6
49. Suyama K, Mizota S, Minagawa T, Hayashi K, Miyazaki H, Nagata I. A ruptured anterior communicating artery aneurysm associated with internal carotid artery agenesis and a middle cerebral artery anomaly. J Clin Neurosci. 2009. 16: 585-6
50. Tanaka Y, Masuzawa T, Kitano I, Matsumoto T. Hypoplasia of the left internal carotid artery associated with anterior communicating aneurysm, intercarotid anastomosis and left rete carotidis. No To Shinkei. 1996. 48: 170-4
51. Tangchai P, Khaoborisut V. Agenesis of internal carotid artery associated with aneurysm of contralateral middle cerebral artery. Neurology. 1970. 20: 809
52. Teal JS, Rumbaugh CL, Bergeron RT, Segali HD. Congenital absence of the internal carotid artery associated with cerebral hemiatrophy, absence of the external carotid artery, and persistence of the stapedial artery. Am J Roentgenol. 1973. 118: 534-45
53. Tracy PT. Unusual intercarotid anastomosis associated with anterior communicating artery aneurysm: Case report. J Neurosurg. 1987. 67: 765-7
54. Tsuruta J. A case of complete absence of the left internal carotid artery associated with an aneurysm of the anterior communicating artery. No Shinkei Geka. 1977. 5: 895-900
55. Waga S, Okada M, Kojima T. Saccular aneurysm associated with absence of the left cervical carotid arteries. Neurosurgery. 1978. 3: 208-12
56. Wani A, Behari S, Lyndoh B, Jaiswal A, Sahu R, Jain V. Multiple aneurysms associated with agenesis of internal carotid artery. Turk Neurosurg. 2011. 21: 83-5
57. Wong GK, Yu SC, Antonio GE, Poon WS. A rare anatomic variant of a solitary internal carotid artery associated with moyamoya phenomenon of the middle cerebral artery. Am J Neuroradiol. 2006. 27: 2012-3
58. Yoshida Y, Tochinai H, Murakami M, Murakami T, Saiki I, Kanaya H. A case of absence of the left internal carotid artery associated with aneurysm of the anterior communicating artery. No Shinkei Geka. 1988. 16: 1089-94
59. Zink WE, Komotar RJ, Meyers PM. Internal carotid aplasia/ hypoplasia and intracranial saccular aneurysms: Series of three new cases and systematic review of the literature. J Neuroimaging. 2007. 17: 141-7