- Department of Neurosurgery, Kesennuma City Hospital, Kesennuma, Miyagi, Japan,
- Department of Neurosurgery, Tohoku University Graduate School of Medicine, Aobaku, Sendai, Miyagi, Japan.
Correspondence Address:
Norio Narita
Department of Neurosurgery, Tohoku University Graduate School of Medicine, Aobaku, Sendai, Miyagi, Japan.
DOI:10.25259/SNI_853_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahito Katsuki1, Dan Ozaki1, Norio Narita1, Naoya Ishida1, Ohmi Watanabe1, Siqi Cai1, Shinya Shimabukuro1, Teiji Tominaga2. Unilateral posterior reversible encephalopathy syndrome characterized with a long and gradually exacerbating course over 3 years and that presented propofol infusion syndrome – A case report. 13-Jan-2021;12:19
How to cite this URL: Masahito Katsuki1, Dan Ozaki1, Norio Narita1, Naoya Ishida1, Ohmi Watanabe1, Siqi Cai1, Shinya Shimabukuro1, Teiji Tominaga2. Unilateral posterior reversible encephalopathy syndrome characterized with a long and gradually exacerbating course over 3 years and that presented propofol infusion syndrome – A case report. 13-Jan-2021;12:19. Available from: https://surgicalneurologyint.com/surgicalint-articles/10520/
Abstract
Background: Posterior reversible encephalopathy syndrome (PRES) is characterized by acute neurological symptoms and vasogenic edema, and most patients wholly recover. We report a unilateral PRES patient characterized by a gradual onset followed by propofol infusion syndrome (PRIS) due to general anesthesia therapy.
Case Description: A 32-year-old woman had ovarian dysfunction treated by Kaufmann’s treatment for 17 years. Three years ago, she developed seizures, and photophobia and myoclonus sometimes occurred. This time, she had strong photophobia and nausea for 3 months and then developed tonic-clonic seizures for 3 min. Her blood pressure and laboratory test on admission were all within normal limits. She presented no neurological deficits at admission, but the T2-weighted image (T2WI) showed a high-intensity area (HIA), and arterial spin labeling (ASL) image described cerebral blood flow (CBF) increase in the left parieto-occipital region. We diagnosed PRES and started anticonvulsants, antihypertensive, and steroid pulse therapy. However, her aphasia and neuroimaging findings worsened, so we started general anesthesia treatment with propofol on day 29. On day 32, she suddenly developed multiple organ dysfunctions due to PRIS. After intensive care with other sedatives over 2 months, the systemic status and neurological symptoms gradually improved almost as before the onset. On day 90, HIA in the T2WI in the lesion became small, and CBF was severely downregulated in the ASL image.
Conclusion: Unilateral PRES’s pathophysiology and the association with the female hormone remain unknown. Some patients undergo gradual onset and long-term courses, and we should care for PRIS during PRES treatment.
Keywords: Kaufmann’s treatment, Ovarian dysfunction, Posterior reversible encephalopathy syndrome, Propofol infusion syndrome, Status epilepticus
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a clinical and neuroradiologic entity that involves an acute onset of neurological symptoms and vasogenic edema. It is characterized by relatively acute-onset symptoms of headache, visual disturbance, altered mental status, and seizures. The neuroimaging typically reveals that patchy areas of vasogenic edema generally occurring bilaterally in the posterior parietal and occipital lobes.[
CASE DESCRIPTION
A 32-year-old woman had ovarian dysfunction and had been undergoing Kaufmann’s treatment for 17 years. Kaufmann’s treatment is the sequential administration of estrogen and progesterone cyclically for a fixed period. In her Kaufmann’s treatment’s 28 day cycle, she had been taking 0.625 mg conjugated estrogens for the first 10 days, both 0.625 mg conjugated estrogens and medroxyprogesterone 5 mg for the next 11 days, and nothing for the rest of the 7 days. Three years ago, she developed seizures with the right upper extremity flexion, left upper extremity extension, and conjugate deviation to the right, which led to systemic convulsion. She was then treated with fosphenytoin, levetiracetam, and midazolam, and the convulsion was relieved. However, photophobia and myoclonus seizures sometimes occur, so she had taken levetiracetam 1000 mg/day as an outpatient. Her symptoms had repeated about once per 3 months, but her blood pressure had been within an optimal range.
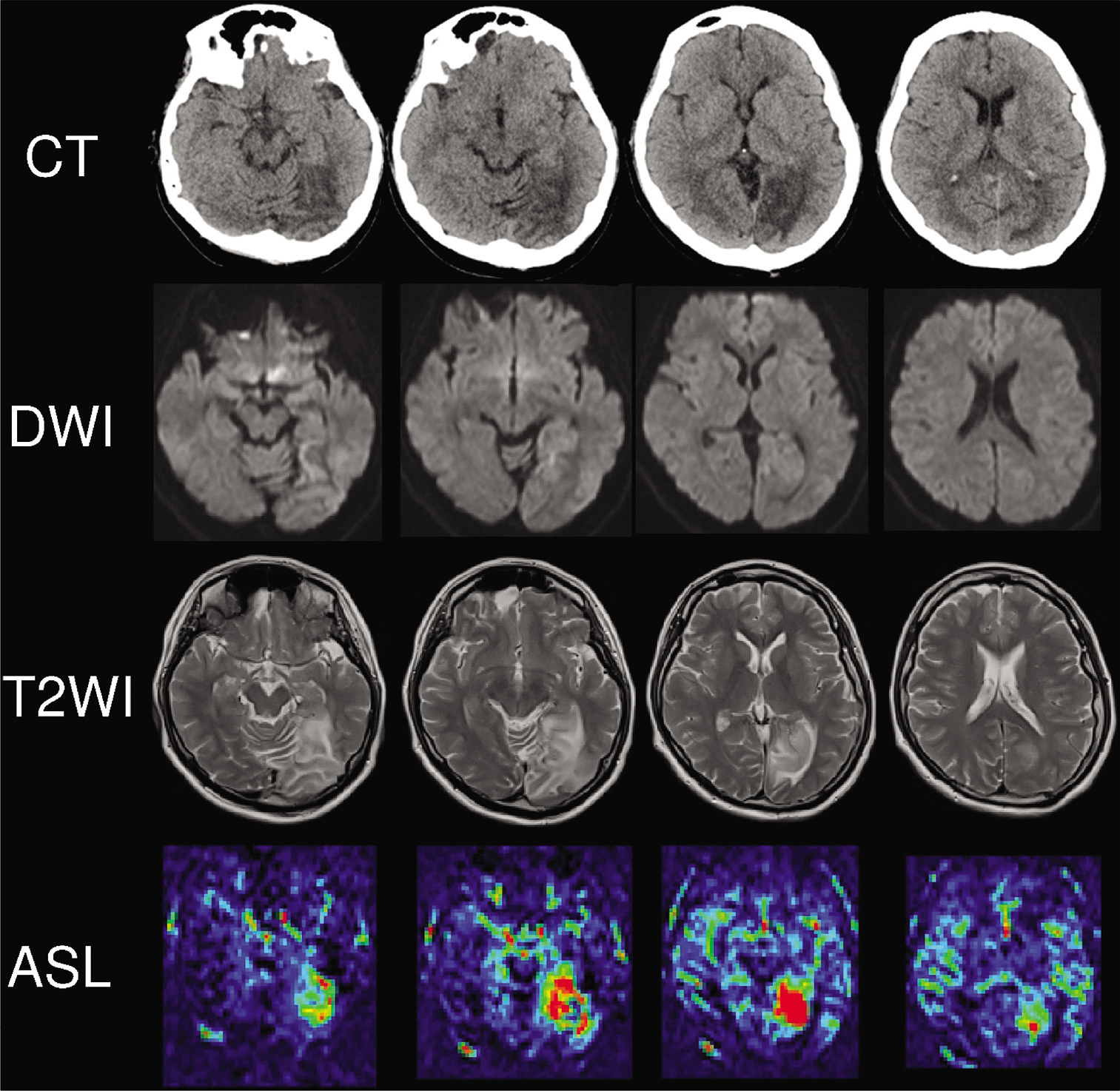
Since 3 months ago in this year, she had been feeling strong photophobia and nausea. She then developed tonic-clonic seizures for 3 min and was transported to our emergency department. Her blood pressure was 124/77 mmHg, and laboratory test results were all within normal limits. She did not present any neurological deficits at admission, but the head computed tomography (CT) revealed a low-density area (LDA) in the left occipital lobe. The diffusion-weighted image (DWI) and T2-weighted image (T2WI) showed high-intensity area (HIA), and arterial spin labeling (ASL) image described cerebral blood flow (CBF) increase in the same region. These findings suggested brain edema [
Figure 2:
Neuroimaging on day 12. Head computed tomography revealed an enlarged low-density area in the left parieto-occipital lobe. The diffusion-weighted image and T2-weighted image also showed the enlarged high-intensity area, and arterial spin labeling image described further cerebral blood flow increase in the same region.
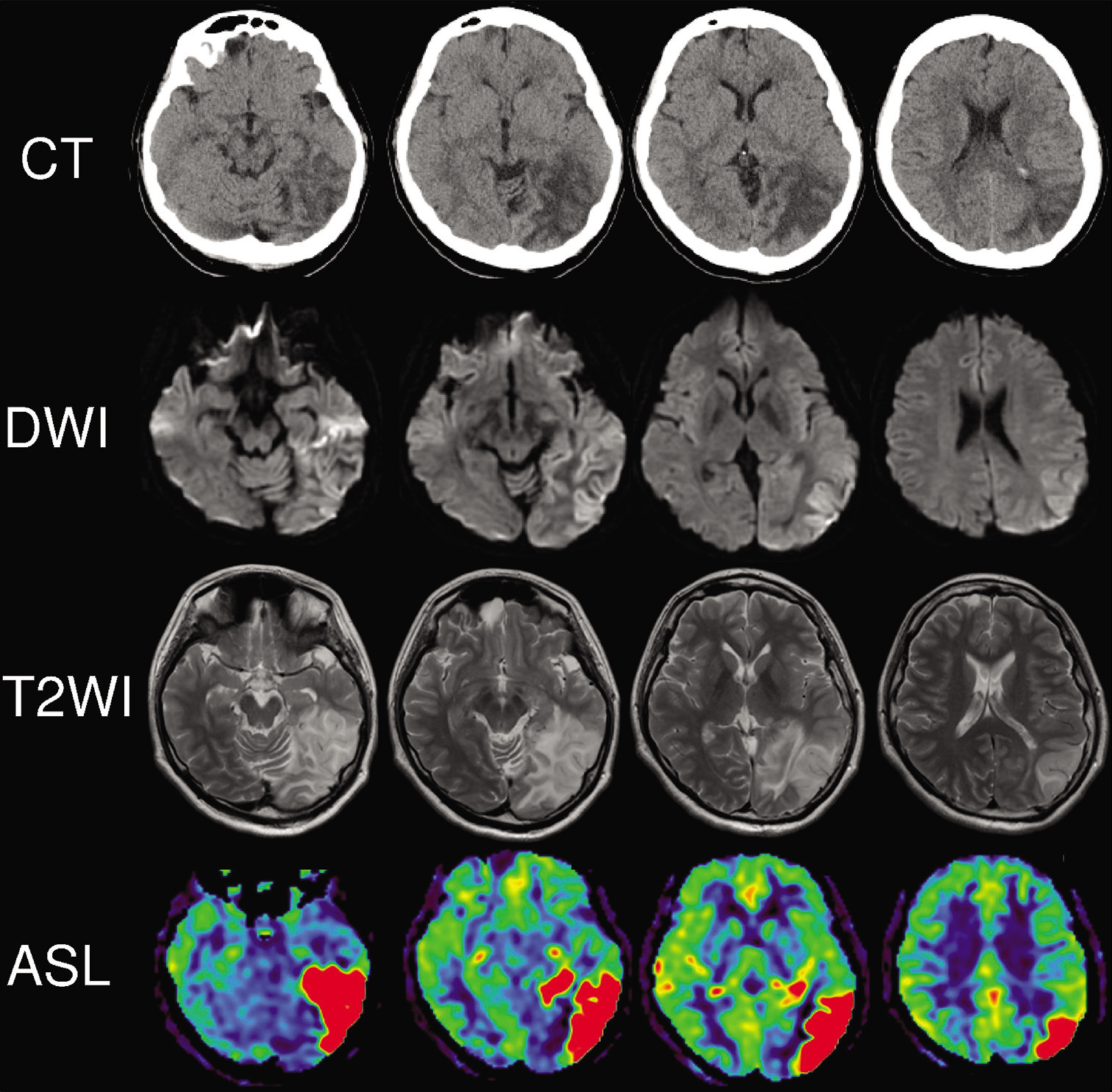
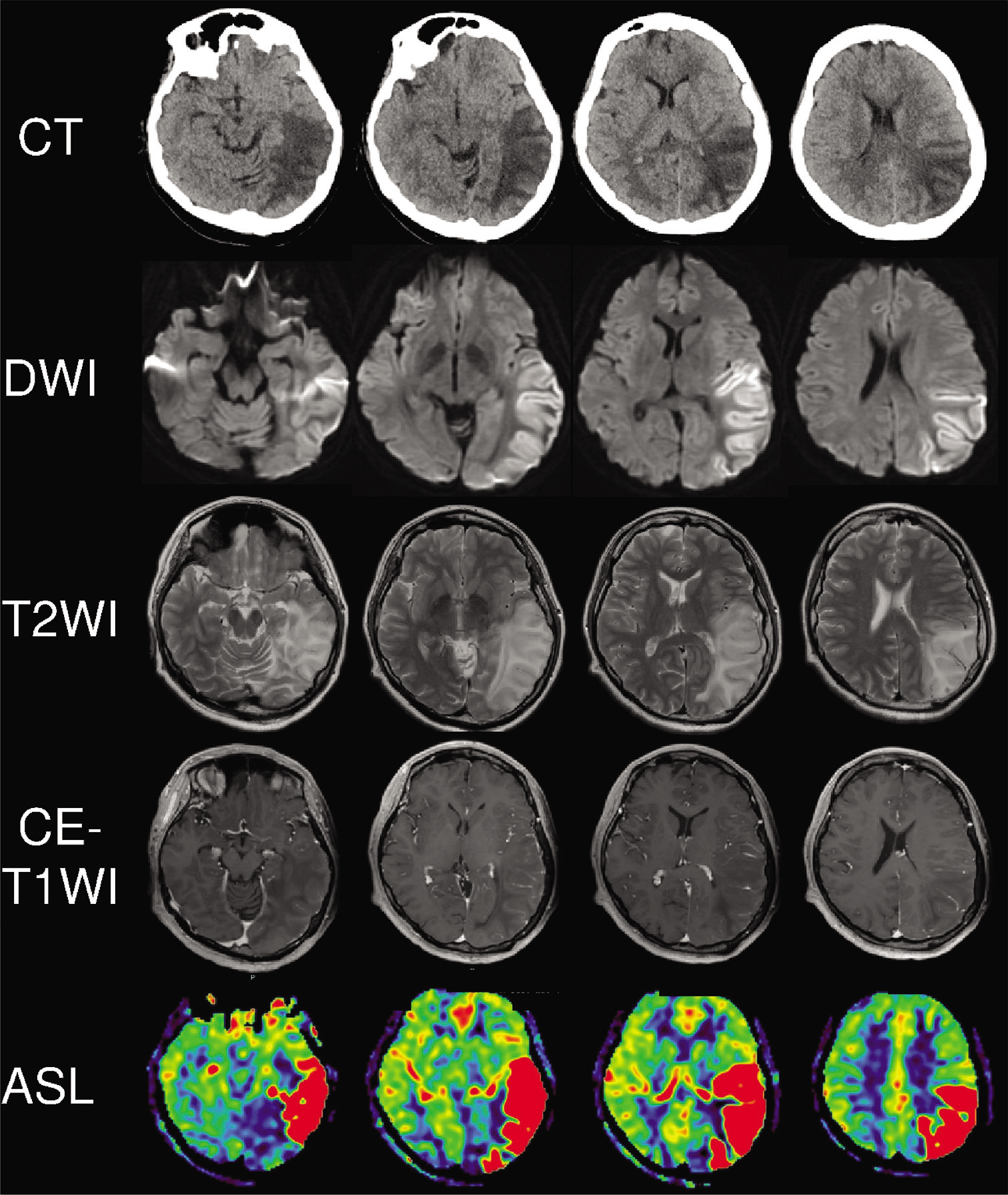
On day 29, the HIA in the T2WI in the left parieto-occipital region enlarged to the temporal lobe, and CBF was further upregulated in the ASL image. Gadolinium-enhanced magnetic resonance imaging was performed, but the lesion was not enhanced; thus, malignant glioma seemed negative [
Figure 3:
Neuroimaging on day 29. Head computed tomography revealed a further enlarged low-density area from the left parieto-occipital lobe to the temporal lobe. The diffusion-weighted image and T2-weighted image also showed the enlarged high-intensity area. Gadolinium-enhanced T1-weighted image reveal that the lesion was not enhanced and arterial spin labeling image described strong cerebral blood flow increase in the same region.
On day 32, she suddenly developed multiple organ dysfunctions with a serum creatinine kinase level of 25,770,000 IU/L. We diagnosed PRIS, and she was transported to another advanced medical care center. After intensive care with vasopressors, continuous hemodiafiltration, and prone position respiratory therapy with sedation by midazolam and dexmedetomidine, her systemic status and consciousness gradually improved. She was extubated on day 68, and continuous hemodiafiltration ended on day 76. Her consciousness finally improved as that before the onset, but intention tremor was present as a subsequent complication. During the intensive care, electroencephalography did not reveal any epileptiform patterns, and laboratory cerebrospinal fluid (CSF) and blood tests associated with autoimmune and inflammatory diseases were all negative; anti-viral antibodies, anti-myelin oligodendrocyte glycoprotein antibody, anti-Nmethyl-D-aspartate receptor antibody, and anti-aquaporin 4 antibody were negative in both CSF and blood serum. Anti-nuclear antibody, anti-double-stranded deoxyribonucleic acid antibody, Ro/La antibody, serine proteinase 3-anti-neutrophil cytoplasmic antibody, and myeloperoxidase-anti-neutrophil cytoplasmic antibody were all negative. Serum C3, C4, and CH50 levels were 72 mg/dL, 44.3 mg/dL, and 39.9 U/mL, respectively. Serum pyruvate and lactate levels were increased to 1.15 mg/dL and 25.7 mg/dL each.
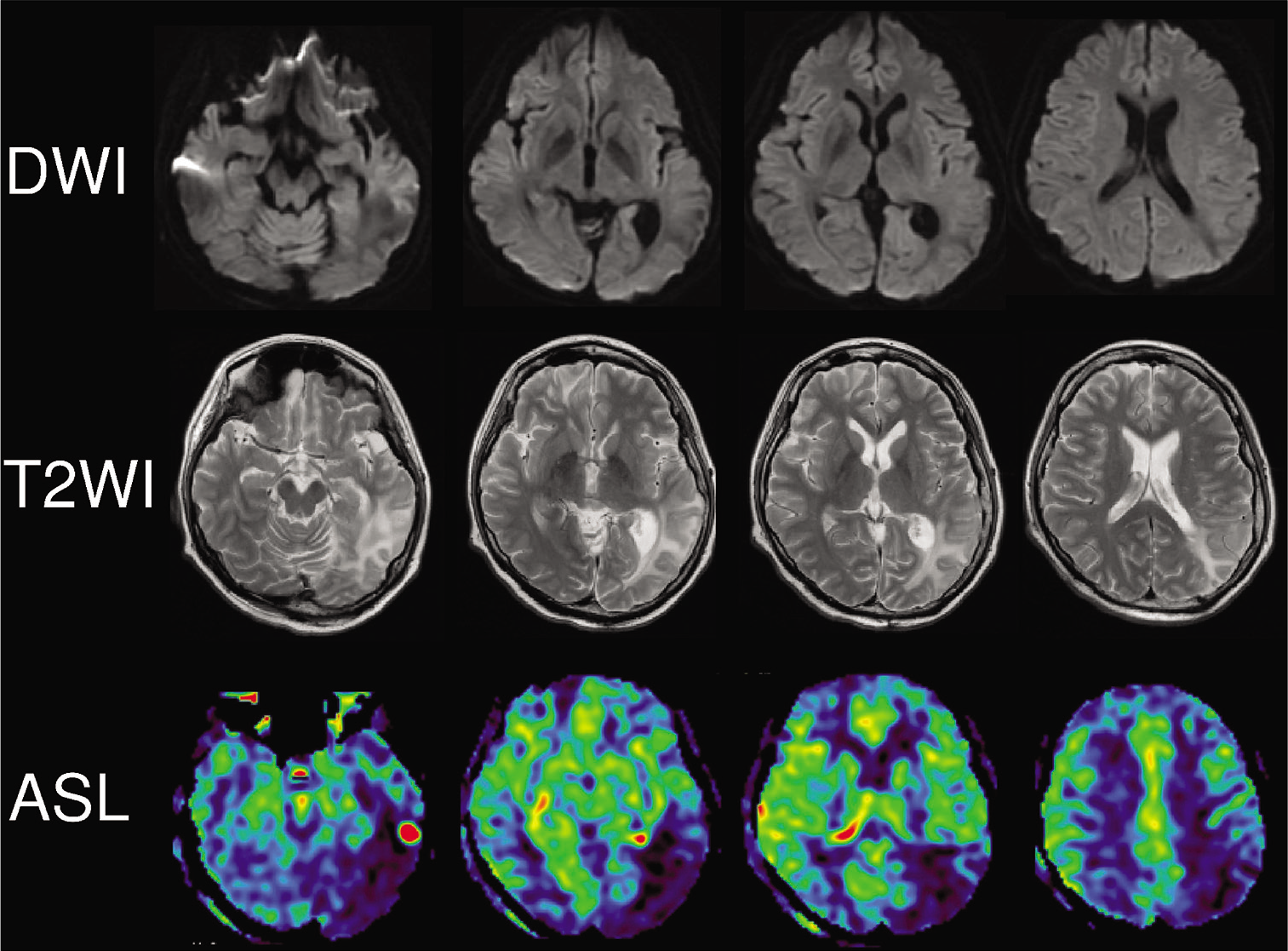
She was transported back to our hospital on day 89 and has continued mainly physical therapy for gait disorder due to disuse syndrome rather than higher cognitive training. Her aphasia and apraxia had already disappeared, and she has walked without any assistance. On day 90, HIA in the T2WI in the parieto-occipital region a bit became small, and CBF was severely downregulated in the ASL image. Magnetic resonance spectroscopy did not reveal the peak of lactate [
DISCUSSION
We herein reported a rare case who developed PRES characterized by a long and gradually exacerbating course over 3 years and needed intensive care for seizures with general anesthesia therapy. She had ovarian dysfunction and had been undergoing Kaufmann’s treatment. In this case, there are five notable points; pathophysiology related to female hormones, unilateral variant PRES, gradual onset and improvement, development of PRIS after PRES, and differential diagnosis.
Pathophysiology
There are two leading theories on PRES’s pathophysiology: hypertension and cerebral hyperperfusion theory, and endothelial dysfunction theory.[
The endothelial dysfunction theory’s common factors are the presence of endogenic (preeclampsia, sepsis, and autoimmune disorders) or exogenic (chemotherapy and immunosuppressive agents) toxins.[
Unilateral variant PRES
As a unique point of our case, she showed unilateral lesions in the left hemisphere. Typically, PRES presented bilateral and symmetrical lesions.[
Gradual onset and prolonged recovery course
PRES’s prognosis is usually favorable, and 75%–90% of patients wholly recover with a mean time to full clinical recovery about 2–8 days.[
Propofol infusion syndrome
Propofol is used for treating refractory status epilepticus. PRIS is a rare but often fatal syndrome characterized by lactic acidosis, lipidemia, and cardiac failure, associated with propofol infusion over prolonged periods. The incidence of PRIS during treatment for status epilepticus is about 39%, and the mortality rate is up to 6%.[
Differential diagnosis
Based on the clinical course and radiological findings, we raised progressive malignant glioma and mitochondrial myopathy encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) as differential diagnoses. The incidence of malignant gliomas is approximately 5/100,000, and malignant gliomas constitute 35–45% of primary brain tumors. Gadolinium-enhanced T1-weighted image usually reveals enhanced lesion in the progressive glioma,[
MELAS is a mitochondrial disorder caused by mutations in the genes in mitochondrial DNA. MELAS can be another differential diagnosis because MELAS and PRIS’s pathophysiological mechanism commonly involves mitochondrial disorder that leads to insufficient energy generation. PRIS patient with the genetically proven mitochondrial disease[
CONCLUSION
We herein reported a rare case who developed unilateral PRES characterized with a not acute but long and gradually exacerbating course over 3 years, followed by PRIS due to general anesthesia therapy for status epilepticus that needed 2-month intensive care. PRES’s pathophysiology remains unknown, and further studies on the association, especially female hormone and PRES, are needed. We also should care for prolonged clinical course and PRIS during PRES treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anderson RC, Patel V, Sheikh-Bahaei N, Liu CS, Rajamohan AG, Shiroishi MS. Posterior reversible encephalopathy syndrome (PRES): Pathophysiology and neuro-imaging. Front Neurol. 2020. 11: 463
2. Brady E, Parikh NS, Navi BB, Gupta A, Schweitzer AD. The imaging spectrum of posterior reversible encephalopathy syndrome: A pictorial review. Clin Imaging. 2018. 47: 80-9
3. Cossette É, Cloutier I, Tardif K, Donpierre G, Tanguay JF. Estradiol inhibits vascular endothelial cells pro-inflammatory activation induced by C-reactive protein. Mol Cell Biochem. 2013. 373: 137-47
4. De Seze J, Mastain B, Stojkovic T, Ferriby D, Pruvo JP, Destée A. Unusual MR findings of the brain stem in arterial hypertension. AJNR Am J Neuroradiol. 2000. 21: 391-4
5. Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017. 264: 1608-16
6. Freilinger T, Schmidt C, Duering M, Linn J, Straube A, Peters N. Reversible cerebral vasoconstriction syndrome associated with hormone therapy for intrauterine insemination. Cephalalgia. 2010. 30: 1127-32
7. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: Clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015. 14: 914-25
8. Garcia JM, Stillings SA, Leclerc JL, Phillips H, Edwards NJ, Robicsek SA. Role of interleukin-10 in acute brain injuries. Front Neurol. 2017. 8: 244
9. Hwang WS, Gwak HM, Seo DW. Propofol infusion syndrome in refractory status epilepticus. J Epilepsy Res. 2013. 3: 21-7
10. Japan Intractable Diseases Information Center Journal Mitochondrial Disease. Available from: https://www.nanbyou.or.jp/entry/335 [Last accessed on 2020 Nov 01].
11. Kao HW, Chiang SW, Chung HW, Tsai FY, Chen CY. Advanced MR imaging of gliomas: An update. Biomed Res Int. 2013. 2013: 970586
12. Kawakita K, Abe Y, Kirizume K, Shinohara N, Kawai N, Tamiya T. Posterior reversible encephalopathy syndrome: Five case reports. Nihon Kyukyu Igakukai Zasshi. 2012. 23: 357-63
13. Khan IR, Pai V, Mundada P, Sitoh YY, Purohit B. Detecting the uncommon imaging manifestations of posterior reversible encephalopathy syndrome (PRES) in adults: A comprehensive illustrated guide for the trainee radiologist. Curr Probl Diagn Radiol. 2020. p.
14. Lee M, Kim TH, Kim SJ, Jee BC. Posterior reversible encephalopathy syndrome in a woman who used gonadotropin-releasing hormone agonists: A case report. Obstet Gynecol Sci. 2019. 62: 69-72
15. Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: The endothelial hypotheses. Med Hypotheses. 2014. 82: 619-22
16. Mayama M, Uno K, Tano S, Yoshihara M, Ukai M, Kishigami Y. Incidence of posterior reversible encephalopathy syndrome in eclamptic and patients with preeclampsia with neurologic symptoms. Am J Obstet Gynecol. 2016. 215: 239.e1-5
17. McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018. 49: 524-30
18. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007. 189: 904-12
19. Mirrakhimov AE, Voore P, Halytskyy O, Khan M, Ali AM. Propofol infusion syndrome in adults: A clinical update. Crit Care Res Pract. 2015. 2015: 260385
20. Oishi C, Okano H, Uegama K, Kobayashi K, Nagane M, Chiba A. Brainstem variant of reversible posterior leukoencephalopathy syndrome with a prolonged clinical course: A case report. Rinsho Shinkeigaku. 2008. 48: 737-41
21. Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993. 362: 801-9
22. Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: Long-term follow-up. J Neurol Neurosurg Psychiatry. 2010. 81: 773-7
23. Savard M, Dupré N, Turgeon AF, Desbiens R, Langevin S, Brunet D. Propofol-related infusion syndrome heralding a mitochondrial disease: Case report. Neurology. 2013. 81: 770-1
24. Shiga Y, Kanaya Y, Kono R, Takeshima S, Shimoe Y, Kuriyama M. Posterior reversible encephalopathy syndrome of the midbrain and hypothalamus-a case report of uremic encephalopathy presenting with hypersomnia. Rinsho Shinkeigaku. 2016. 56: 43-7
25. Shimizu J, Tabata T, Tsujita Y, Yamane T, Yamamoto Y, Tsukamoto T. Propofol infusion syndrome complicated with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes: A case report. Acute Med Surg. 2020. 7: e473
26. Tlemsani C, Mir O, Boudou-Rouquette P, Huillard O, Maley K, Ropert S. Posterior reversible encephalopathy syndrome induced by anti-VEGF agents. Target Oncol. 2011. 6: 253-8






Masahito Katsuki
Posted February 23, 2021, 5:19 pm
The propofol described in this article is 1% Propofol Inj. Maruishi.