- Department of Hematology and Hematologic Malignancies, University of Utah, Salt Lake, Utah,
- Department of Neurosurgery, Tufts Medical Center, Washington, Boston, United States.
- Department of Pathology and Laboratory Medicine, Tufts Medical Center, Washington, Boston, United States.
- Department of John Conant Davis Myeloma and Amyloid Program, Tufts Medical Center, Washington, Boston, United States.
- Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, New York, United States.
Correspondence Address:
Amandeep Godara, MD, Division of Hematology and Hematologic Malignancies, University of Utah, Salt Lake City, Utah, United States.
DOI:10.25259/SNI_1235_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Amandeep Godara1, Andy Y. Wang2, Knarik Arkun3, Teresa Fogaren4, Adnan S. Qamar3, Ellen D. McPhail5, James Kryzanski2, Ron Riesenburger2, Raymond Comenzo4. Unraveling a rare cause of spinal stenosis: Coexistent AL and ATTR amyloidosis involving the ligamentum flavum. 12-Jan-2022;13:12
How to cite this URL: Amandeep Godara1, Andy Y. Wang2, Knarik Arkun3, Teresa Fogaren4, Adnan S. Qamar3, Ellen D. McPhail5, James Kryzanski2, Ron Riesenburger2, Raymond Comenzo4. Unraveling a rare cause of spinal stenosis: Coexistent AL and ATTR amyloidosis involving the ligamentum flavum. 12-Jan-2022;13:12. Available from: https://surgicalneurologyint.com/surgicalint-articles/11340/
Abstract
Background: Amyloidosis is a protein misfolding disorder that leads to the deposition of beta-pleated sheets of a fibrillar derivative of various protein precursors. Identification of the type of precursor protein is integral in treatment decision-making. The presence of two different types of amyloid in the same patient is unusually rare, and there are no previous reports of two different types of amyloid deposition in the ligamentum flavum (LF) in the same patient.
Case Description: Here, we describe two patients with spinal stenosis who underwent laminectomies and were found to have AL and ATTR amyloid deposits in the LF.
Conclusion: As the spine is becoming recognized as a site for ATTRwt amyloid deposition, patients undergoing spinal decompression surgery may potentially benefit from evaluation for amyloidosis in the LF.
Keywords: Ligamentum flavum, Light chain, Spinal stenosis, Wild-type transthyretin amyloid
INTRODUCTION
Systemic light chain (AL) amyloidosis is one of the most common types of amyloidosis, although over 30 other proteins are implicated in this rare disease.[
CASE DESCRIPTION
Patient 1
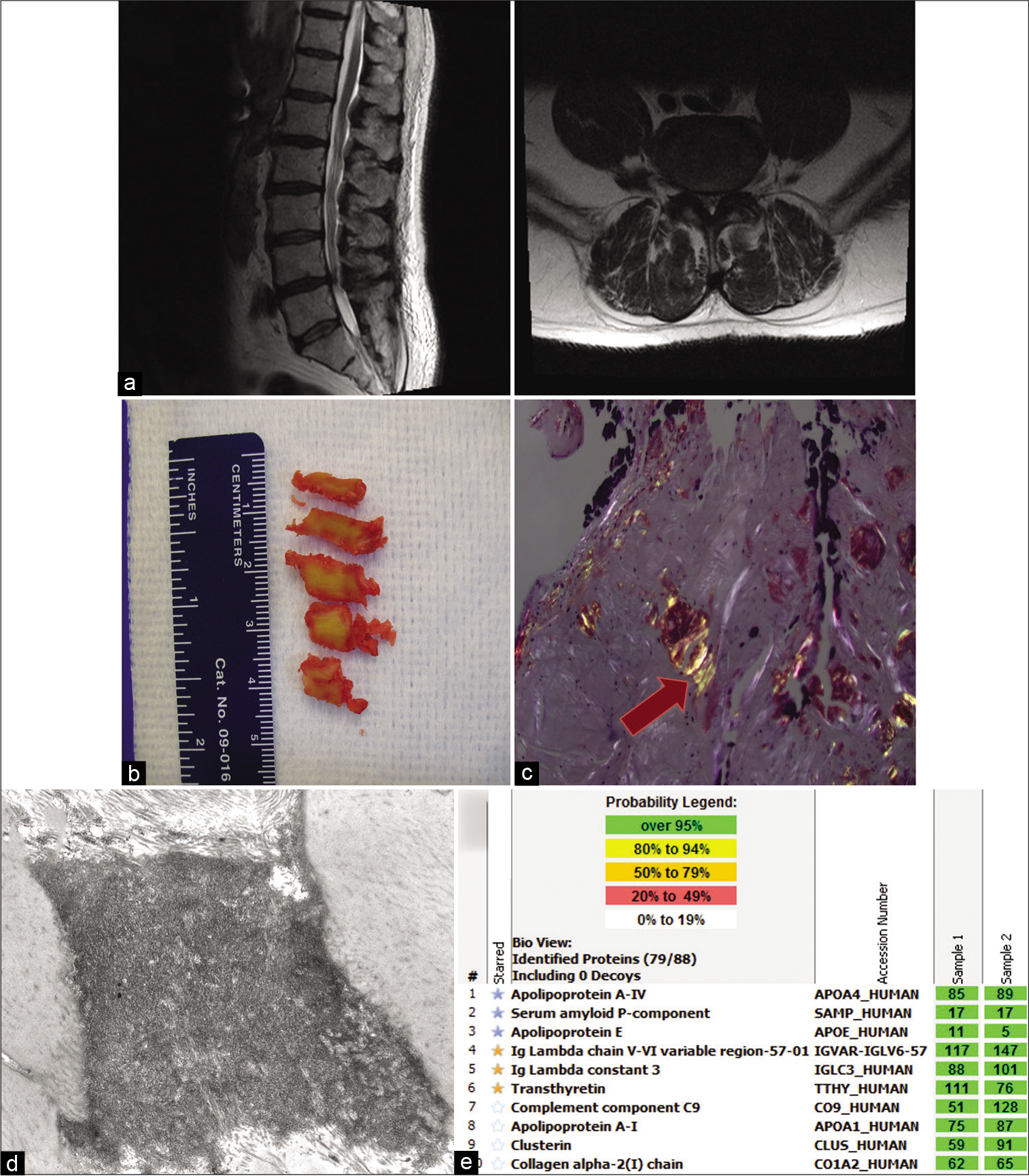
A 69-year-old man with 4 years of smoldering IgG lambda myeloma was seen for concern of systemic light-chain amyloidosis due to macroglossia, worsening fatigue, and unintentional weight loss. He had a prior history of C3-T1 cervical laminectomy and decompression with fusion 2 years prior. An abdominal fat pad aspirate showed focal congophilia and faint birefringence under polarized light. Bone marrow biopsy showed involvement with 30–40% plasma cells and amyloid deposits by Congo red staining. Over the prior year, he had developed progressive lower back pain and required the use of a wheelchair. Magnetic resonance imaging (MRI) of the spine showed severe spinal stenosis at L4-L5. He underwent posterior L4-L5 laminectomies, medial facetectomies, and decompression of the thecal sac [
Figure 1:
Imaging and pathology for Patient 1. (a) T2-weighted preoperative magnetic resonance imaging; left: sagittal view, right: axial view at the L4-L5 level showing thickened ligamentum flavum. (b) Gross ligamentum flavum specimens resected from surgery. (c) Congo red stains under polarized light, showing apple green birefringence (indicated by arrow). (d) Electron microscopy of ligamentum flavum showing amyloid deposit. (e) Liquid chromatography tandem mass spectrometry (LC–MS/MS) performed on peptides extracted from Congo red-positive microdissected areas of paraffin-embedded specimen, peptide profile consistent with ATTR (transthyretin)-type amyloid and AL (lambda)-type amyloid. Yellow stars indicate the amyloid precursor proteins (transthyretin and lambda light chains) and blue stars indicate the universal amyloid proteins (apolipoprotein A-IV, serum amyloid P-component, and apolipoprotein E). The total number of MS/MS spectra matching to a protein in a sample is shown in the green boxes. Two independent samples were analyzed per case.
Patient 2
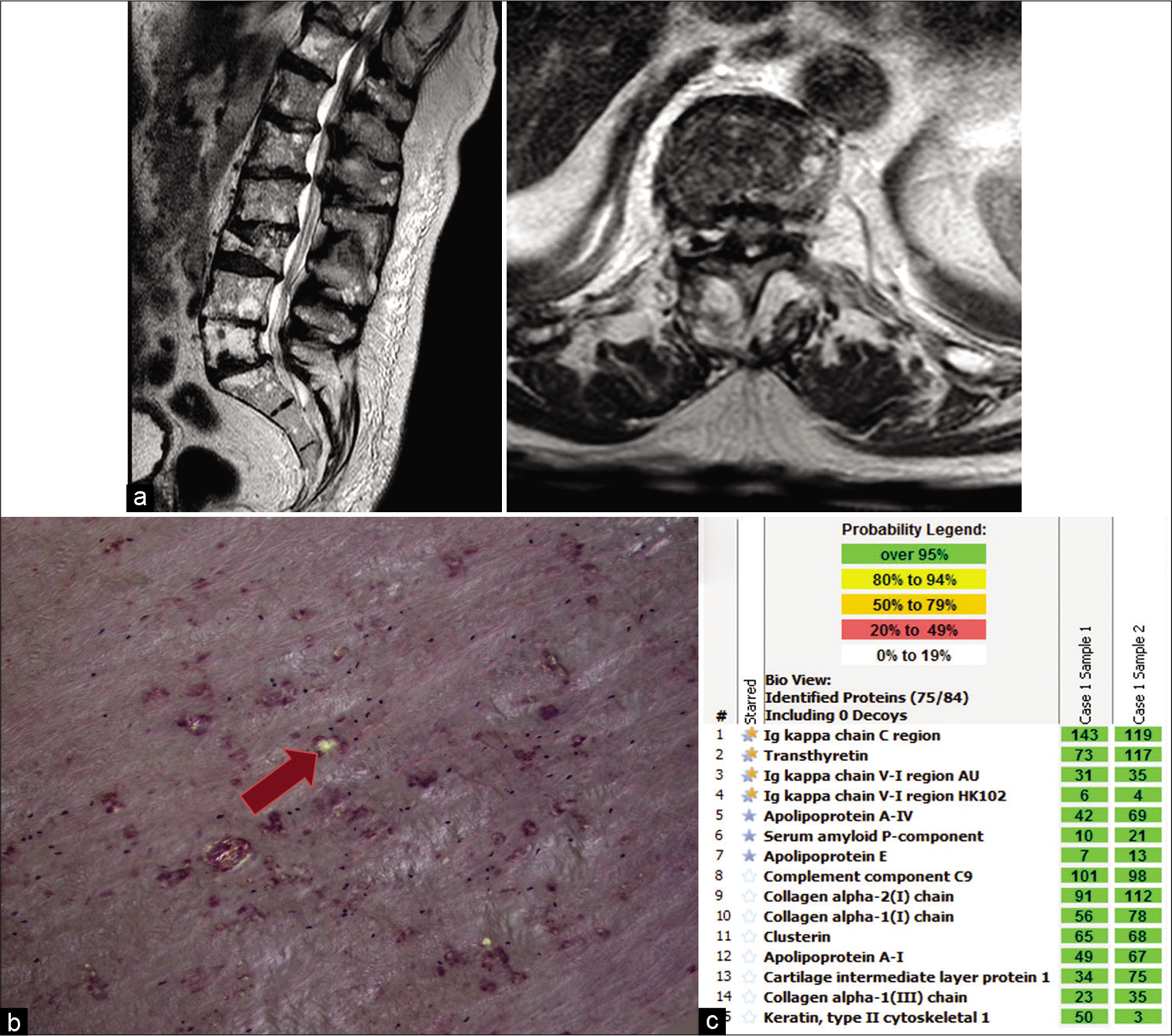
A 76-year-old male with a known diagnosis of IgG kappa multiple myeloma with skeletal involvement for the past 8 years had been previously treated with RVD (lenalidomide, bortezomib, and dexamethasone) and Id (ixazomib and dexamethasone). Nine months before his surgery, he had disease progression with new bone lesions, for which he was started on pomalidomide and dexamethasone. At that time, he was also found to have atrial fibrillation and new-onset systolic heart failure with an ejection fraction of 45% on echocardiogram. He was evaluated for worsening lower back pain and MRI showed loss of height at T10 and mild retropulsion of the posterior aspect of the T6 and T10 vertebral bodies causing neuroforaminal stenosis at T10-T11 and T11-T12 [
Figure 2:
Imaging and pathology for Patient 2. (a) T2-weighted preoperative magnetic resonance imaging; left: sagittal view, right: axial view at T10-T11 level showing thickened ligamentous flavum. (b) Congo red stains under polarized light, showing apple green birefringence (indicated by arrow). (c) Liquid chromatography tandem mass spectrometry (LC–MS/MS) performed on peptides extracted from Congo red-positive microdissected areas of paraffin-embedded specimen detected a peptide profile consistent with ATTR (transthyretin)-type amyloid and AL (kappa)-type amyloid. Blue/yellow stars indicate the amyloid precursor proteins (transthyretin and kappa light chains) and blue stars indicate the universal amyloid proteins (apolipoprotein A-IV, serum amyloid P-component, and apolipoprotein E). The total number of MS/ MS spectra matching to a protein in a sample is shown in the green boxes. Two independent samples were analyzed per case.
He underwent an uneventful decompressive T10-T11 laminectomy; the excised LF was a 3 × 1.5 × 0.5 cm aggregate of pink-red fibrous rubbery tissue. Congo red stain showed multifocal congophilic deposits that had apple green birefringence under polarized light [
DISCUSSION
The two patients described above presented with spinal stenosis and surgically resected LF showed amyloid deposits that were derived from both circulating free light chains and transthyretin protein. Both patients had symptoms of rapidly progressive myelopathy, which suggests that the AL amyloid deposited simultaneously in the spine as other tissues and was a contributing cause to spinal stenosis. These findings present a unique problem for a rare disorder as more than 1 type of amyloidosis in the same patient is rarely encountered.
Spinal stenosis is a progressive degenerative condition that results in significant morbidity and disability affecting up to 20% of persons above the age of 60.[
Our two cases raise the question of whether the presence of ATTR amyloid in the LF provides a nidus for seeding and propagation for other types of amyloid fibrils. The significance of these findings requires careful contemplation as the presence of ATTR amyloid in the spinal ligament might represent a localized form of amyloidosis which may precede the development of cardiac amyloidosis as is the case with carpal tunnel syndrome.[
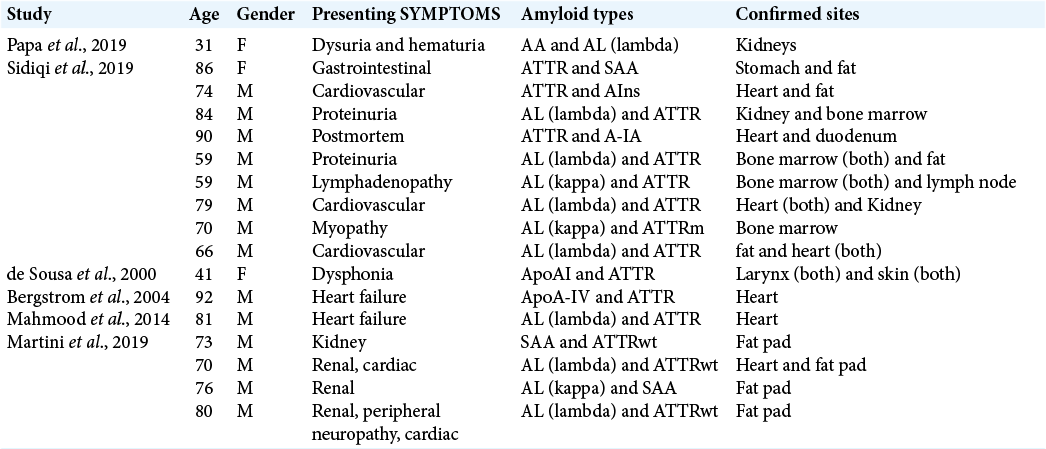
Review of literature for previously reported cases of two different types of amyloidosis in the same patient is provided in [
As survival improves for patients with AL amyloidosis, it is possible that some of them would also develop ATTRwt amyloidosis later in life.[
CONCLUSION
As the spine is increasingly being recognized as a site for ATTRwt amyloid deposition, patients with plasma cell disorders undergoing surgery for spinal stenosis may potentially benefit from evaluation for amyloidosis in the LF. If amyloid deposits are noted, identification of the type of amyloidosis is important as several different types of amyloid can occur simultaneously in the same tissue. Recognizing the types of amyloid provides the opportunity to screen for the involvement of other body sites with subsequent therapeutic implications.
Declaration of patient consent
Patient consent not required as patient identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aus dem Siepen F, Hein S, Prestel S, Baumgartner C, Schonland S, Hegenbart U. Carpal tunnel syndrome and spinal canal stenosis: Harbingers of transthyretin amyloid cardiomyopathy?. Clin Res Cardiol. 2019. 108: 1324-30
2. Beamer YB, Garner JT, Shelden CH. Hypertrophied ligamentum flavum. Clinical and surgical significance. Arch Surg. 1973. 106: 289-92
3. Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJ, Sekijima Y. Amyloid nomenclature 2020: Update and recommendations by the international society of amyloidosis (ISA) nomenclature committee. Amyloid. 2020. 27: 217-22
4. Bergström J, Murphy CL, Weiss DT, Solomon A, Sletten K, Hellman U. Two different types of amyloid deposits--apolipoprotein A-IV and transthyretin--in a patient with systemic amyloidosis. Lab Invest. 2004. 84: 981-8
5. Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: Contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001. 98: 714-20
6. de Sousa MM, Vital C, Ostler D, Fernandes R, PougetAbadie J, Carles D. Apolipoprotein AI and transthyretin as components of amyloid fibrils in a kindred with apoAI Leu178His amyloidosis. Am J Pathol. 2000. 156: 1911-7
7. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010. 303: 1259-65
8. Eldhagen P, Berg S, Lund LH, Sörensson P, Suhr OB, Westermark P. Transthyretin amyloid deposits in lumbar spinal stenosis and assessment of signs of systemic amyloidosis. J Intern Med. 2021. 289: 895-905
9. Genevay S, Atlas SJ. Lumbar spinal stenosis. Best Pract Res Clin Rheumatol. 2010. 24: 253-65
10. George KM, Hernandez NS, Breton J, Cooper B, Dowd RS, Nail J. Increased thickness of lumbar spine ligamentum flavum in wild-type transthyretin amyloidosis. J Clin Neurosci. 2021. 84: 33-7
11. Godara A, Riesenburger RI, Zhang DX, Varga C, Fogaren T, Siddiqui NS. Association between spinal stenosis and wild-type ATTR amyloidosis. Amyloid. 2021. 28: 226-33
12. Mahmood S, Gilbertson JA, Rendell N, Whelan CJ, Lachmann HJ, Wechalekar AD. Two types of amyloid in a single heart. Blood. 2014. 124: 3025-7
13. Martini F, Buda G, De Tata V, Galimberti S, Orciuolo E, Masini M. Different types of amyloid concomitantly present in the same patients. Hematol Rep. 2019. 11: 7996
14. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019. 12: e006075
15. Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: Cracking the glass ceiling of early death. Blood. 2017. 129: 2111-9
16. Papa R, Gilbertson JA, Rendell N, Wechalekar AD, Gillmore JD, Hawkins PN. Two types of systemic amyloidosis in a single patient. Amyloid. 2020. 27: 275-6
17. Prokaeva T, Spencer B, Kaut M, Ozonoff A, Doros G, Connors LH. Soft tissue, joint, and bone manifestations of AL amyloidosis: Clinical presentation, molecular features, and survival. Arthritis Rheum. 2007. 56: 3858-68
18. Sidiqi MH, McPhail ED, Theis JD, Dasari S, Vrana JA, Drosou ME. Two types of amyloidosis presenting in a single patient: A case series. Blood Cancer J. 2019. 9: 30
19. Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018. 72: 2040-50
20. Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009. 114: 4957-9
21. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016. 387: 2641-54
22. Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Pathogenesis and morphologic and immunohistochemical observation. Spine (Phila Pa 1976). 1992. 17: 1353-60