- Department of Neurosurgery, Hospital Clínica Kennedy, Guayaquil, Ecuador
- Department of Neurosurgery, National Institute of Neurology and Neurosurgery Manuel Velasco Suarez, Mexico City, Mexico,
- Department of Medicine, Universidad Espíritu Santo, Samborondon, Ecuador
- Department of General Surgery, Hospital Clínica Kennedy, Guayaquil, Ecuador.
Correspondence Address:
Xavier Wong Achi, Department of Neurosurgery, National Institute of Neurology and Neurosurgery Manuel Velasco Suarez, Insurgentes Sur Av. 3877, Postal Code 14269, Mexico City, Mexico.
DOI:10.25259/SNI_191_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jimmy Achi1, Xavier Wong Achi2, Paula Veintimilla3, Janina Cueva4. Unusual extraneural metastasis of glioblastoma. 23-Jun-2023;14:218
How to cite this URL: Jimmy Achi1, Xavier Wong Achi2, Paula Veintimilla3, Janina Cueva4. Unusual extraneural metastasis of glioblastoma. 23-Jun-2023;14:218. Available from: https://surgicalneurologyint.com/surgicalint-articles/12374/
Abstract
Background: Glioblastoma (GB) is the most common and aggressive malignant brain tumor in adults. Extracranial metastases are very rare, been described in the lungs, soft tissue, or the intraspinal space.
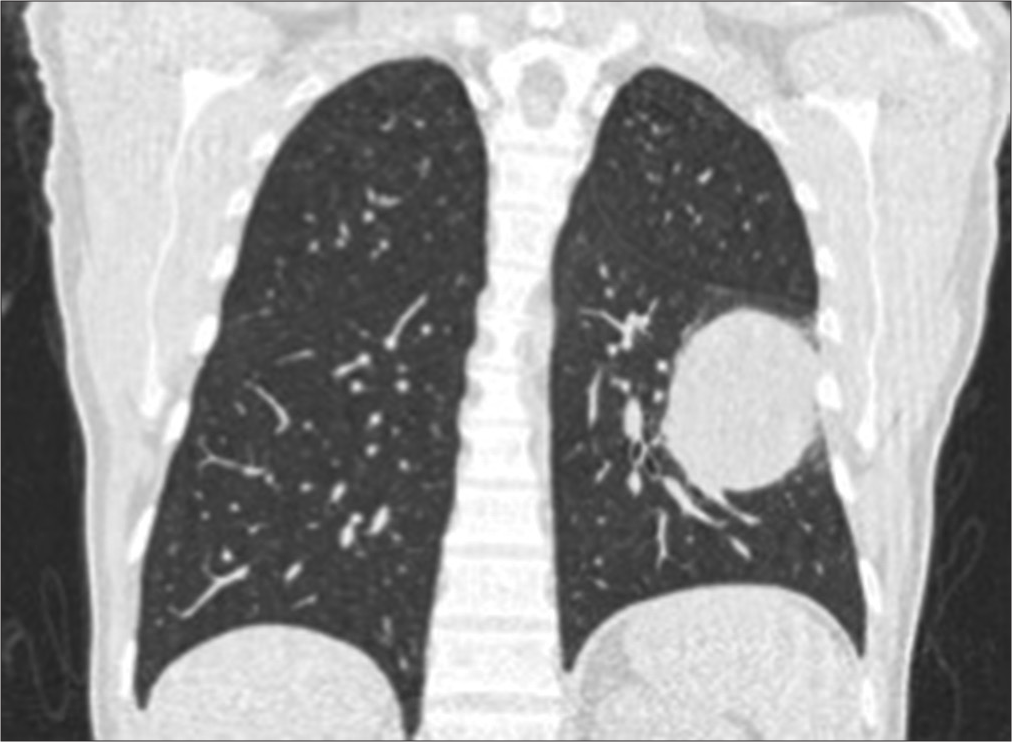
Case Description: Through a PubMed-based bibliographic search, the authors reviewed the cases reported in the literature to date, emphasizing the epidemiology and pathophysiology of this rare condition. A clinical case of a 46-year-old man with an initial diagnosis of gliosarcoma, who received complete surgical and adjuvant treatment and later recurred as GB with incidental finding of a lung tumor, whose pathology reported metastasis of the primary, is illustrated.
Conclusion: Understanding the pathophysiology, it is likely that the incidence of extraneural metastases may continue to increase. Considering improvements in diagnostic techniques that allow early diagnosis, as well as advances in neurosurgical therapy and multimodal management with the aim of improving patient survival, the period in which malignant cells can spread and form extracranial metastases could increase. When screening should be performed to detect metastases in these patients is still not clear. The neuro-oncologists should pay attention to the systematic survey for extraneural metastasis of the GB. Timely detection and early treatment improve overall quality of patients’ life.
Keywords: Extracranial metástasis, Glioblastoma, Gliosarcoma, Tumor
INTRODUCTION
Glioblastoma (GB, World Health Organization [WHO] Grade IV) is the most common primary brain tumor in adults, accounting for 45% of malignant primary central nervous system tumors.[
ILLUSTRATIVE CASE
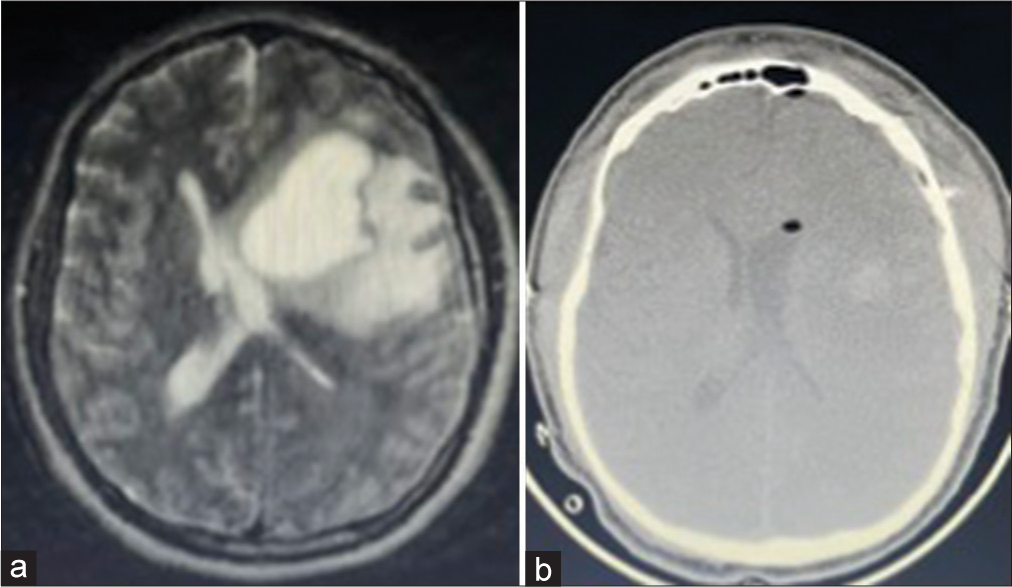
A 46-year-old man with no other medical history was referred after presenting recurrent severe headache, dizziness, and dysarthria of 3 months evolution. A contrast-enhanced magnetic resonance study (MRI) showed an 8-cm intraaxial frontoparietal mass on the left side of the brain, with significant vasogenic edema causing mass effect and anatomical distortion of the lateral ventricles [
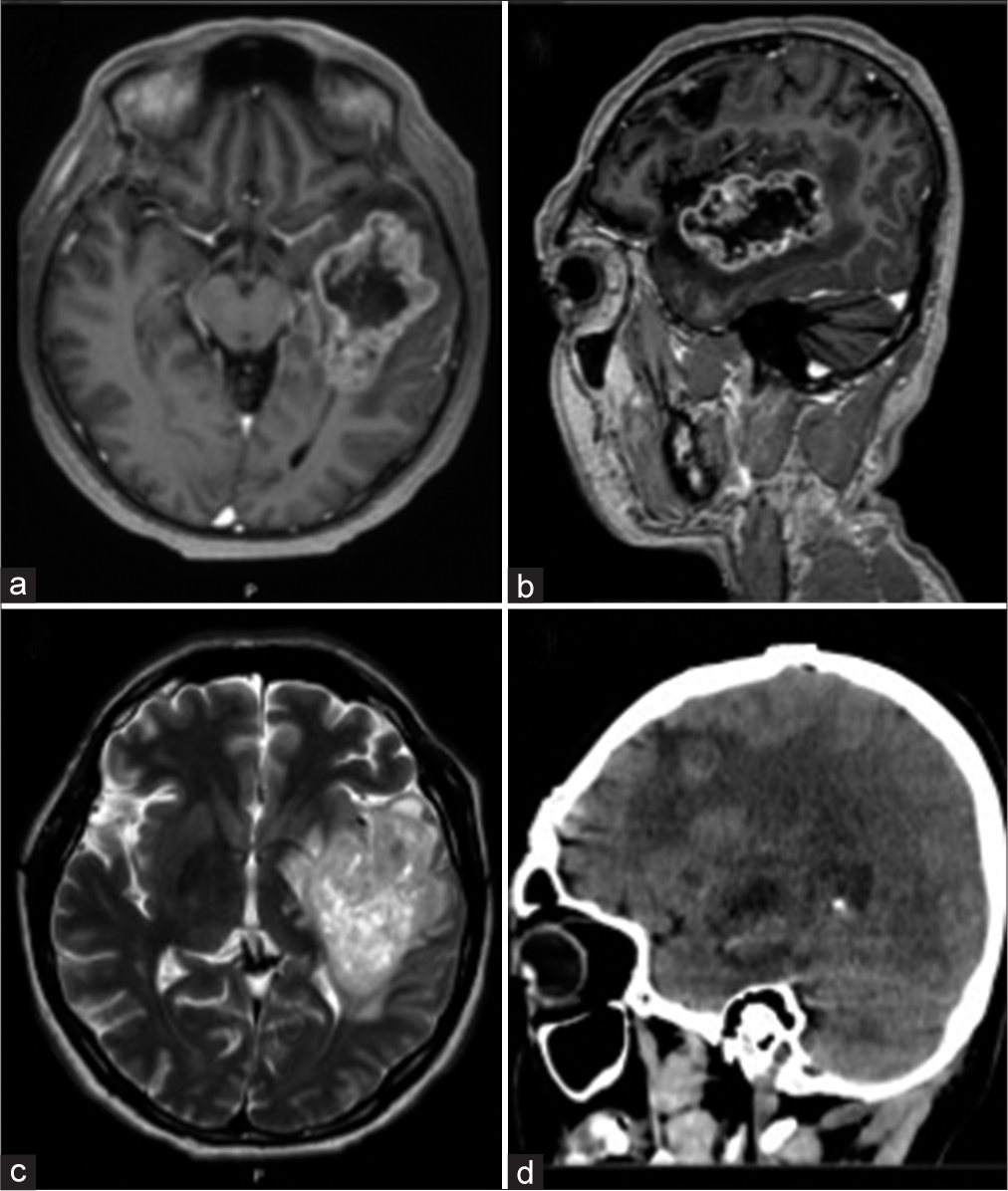
Two years after diagnosis, he presented to the emergency department with seizures. A control MRI revealed a new lesion in the left temporal area with a 5 cm diameter [
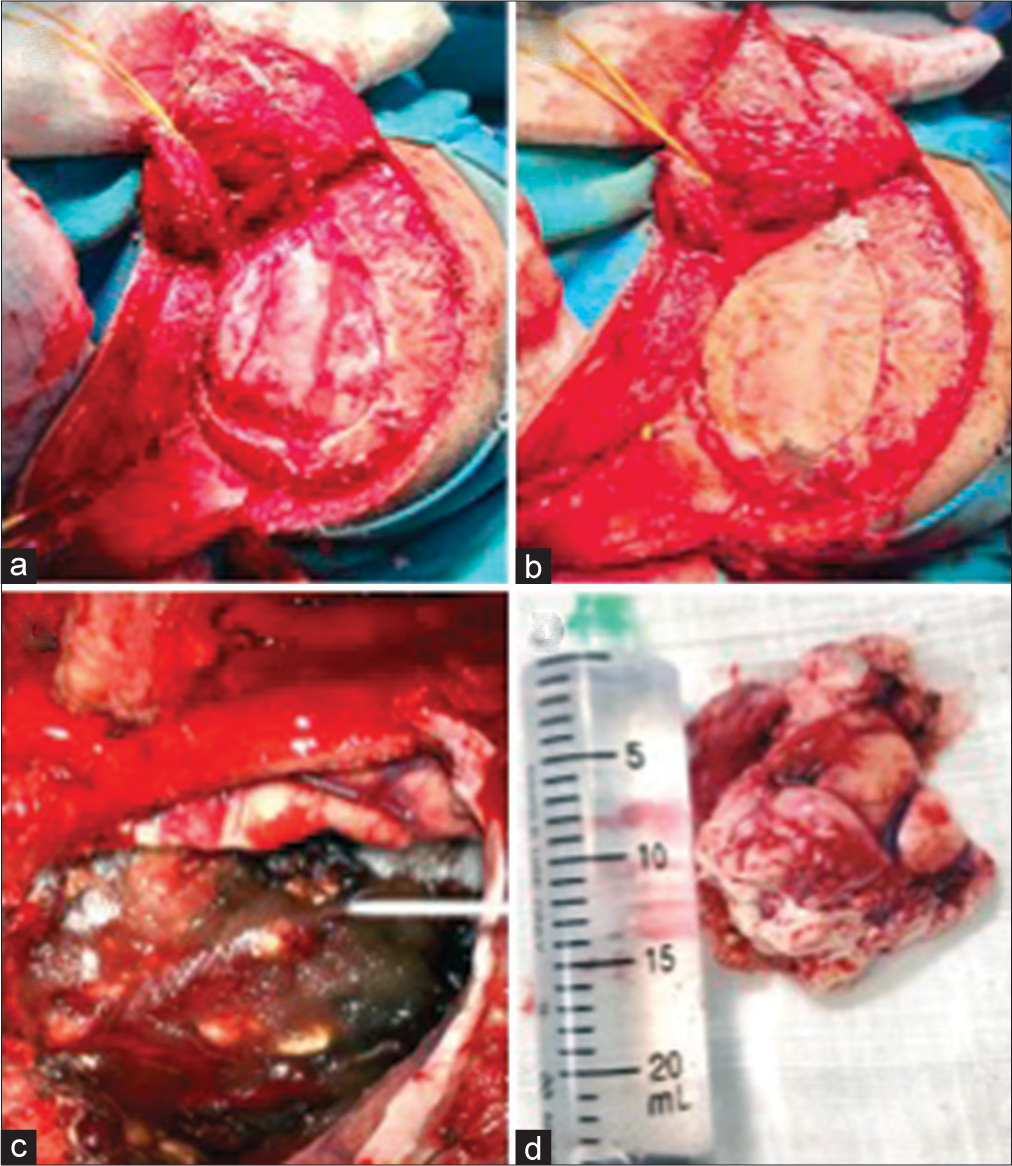
Figure 4:
(a and b) During the second surgical intervention, an extended frontotemporal approach was performed to obtain a better visualization of the tumor and subsequent cranioplasty. (c) After resecting the tumor, due to its malignant characteristics and high vascularization, bleeding was controlled with hemostatic agents. (d) The surgical specimen that after pathological analysis reported a glioblastoma.
DISCUSSION
GB and GS are high grade malignant gliomas defined by the WHO as Grade IV astrocytomas.[
Both tumors are clinically similar affecting mainly 50–70-year-old adults, predominantly men. Furthermore, both tumors show similarly poor survival outcomes (GS 9 months compared to a median 15-month survival for other forms of GB)[
GS can be divided into primary or secondary forms, with the primary form being the most common, while the secondary forms arise after recurrence of a classic GB.[
Extracranial dissemination of both tumors is very rare (0.5% of cases); GS has been described as having a greater propensity to metastasize, but mechanism behind this infrequent condition remains unclear.[
There are several postulates in the literature to explain extraneural dissemination: (1) lymphatic spread through the participation of meningeal lymphatics; (2) hematogenous spread through the intratumoral vascular network that characterizes high-grade gliomas, with invasion of the endothelium and connective tissue. The affinity of sarcomatous neoplasms to spread through this route corroborates the greater potential for metastasis of GS; (3) Dissemination through cerebrospinal fluid.[
At present, GB is still considered one of the most difficult tumors to treat. Maximal safe resection is the cornerstone of treatment, followed by postoperative radiotherapy plus concomitant and adjuvant chemotherapy continues to be the standard treatment.[
CONCLUSION
We report an unusual case of secondary GS metastasis. We believe that possibly the incidence of extraneural metastases may continue to increase, since the pathophysiology of this disease (GB) is better understood, added to innovative surgical techniques aided by neuronavigation, intraoperative neuroelectrophysiologic assessment, and the use of fluorescent materials, better imaging techniques, and multimodal management. Considering the latter whose objective is to improve patient survival, we believe that the period in which malignant cells can spread and form extracranial metastases could increase. When screening should be performed to detect metastases in these patients is still not clear, however, we believe that attention should be given to the systematic study of GB extraneural metastases, especially in those patients with a known GB who have survived a significant period following their initial diagnosis and present with extraneural symptoms with no other explainable cause, or their primary tumor recurs following treatment. Timely detection and early treatment will improve the general quality of life of patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aldoghachi AF, Aldoghachi AF, Breyne K, Ling KH, Cheah PS. Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience. 2022. 491: 240-70
2. Bouwens van der Vlis T, Kros JM, Mustafa DA, van Wijck RT, Ackermans L, van Hagen PM. The complement system in glioblastoma multiforme. Acta Neuropathol Commun. 2018. 6: 91
3. Capion T, Hauerberg J, Broholm H, Muhic A. Multiple extracranial metastases from primary gliosarcoma in a patient with two previous different primary cancers. Case Rep Oncol Med. 2019. 2019: 7849616
4. Choi MG, Lee JH, Lee MS, Suh SJ, Lee YS, Kang DG. Primary gliosarcoma with extracranial metastasis. Brain Tumor Res Treat. 2020. 8: 53-6
5. Da Cunha ML, Maldaun MV. Metastasis from glioblastoma multiforme: A meta-analysis. Rev Assoc Med Bras (1992). 2019. 65: 424-33
6. Javadi AE, Tabriz HM, Zandnejadi A. Postoperative extra-cranial metastasis of glioblastoma: A case report. Iran J Pathol. Winter 2021. 16: 90-4
7. Kanderi T, Gupta V, editors. Glioblastoma multiforme. StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK558954 [Last accessed on 2022 Sep 12]
8. Krivosheya D, Maldaun MV, Prabhu SS, Somasundaram K, editors. Maximal safe resection in glioblastoma: Use of adjuncts. Advances in Biology and Treatment of Glioblastoma: Current Cancer Research. Cham: Springer; 2017. p.
9. Li J, Zhao Y, Tian S, Xu C, Cai Y, Li K. Genetic alteration and clonal evolution of primary glioblastoma into secondary gliosarcoma. CNS Neurosci Ther. 2021. 27: 1483-92
10. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
11. Luo M, Yang J, Sun J, Wang F, Chai X. Primary gliosarcoma with widespread extracranial metastases-spatiotemporal morphological variation. Chin Neurosurg J. 2022. 8: 20
12. Seo Y, Cho W, Kang D, Cha S. Extraneural metastasis of glioblastoma multiforme presenting as an unusual neck mass. J Korean Neurosurg Soc. 2012. 51: 147-50
13. Sibanda Z, Farahani N, Ogbonnaya E, Albanese E. Glioblastoma multiforme: A rare case of spinal drop metastasis. World Neurosurg. 2020. 144: 24-7
14. Swinnen J, Gelin G, Fransis S, Vandevenne J, Van Cauter S. Glioblastoma with extracranial parotid, lymph node, and pulmonary metastases: A case report. Radiol Case Rep. 2019. 14: 1334-47
15. Türkeş G, Parmaksız E, Kıral N, Doğan C, Sağmen S, Fidan A. A rare cause of lung metastasis-glioblastoma multiforme. South Clin Ist Euras. 2018. 29: 203-5
16. Undabeitia J, Castle M, Arrazola M, Pendleton C, Ruiz I, Úrculo E. Multiple extraneural metastasis of glioblastoma multiforme. An Sist Sanit Navar. 2015. 38: 157-61
17. Veerwal H, Meena A, Dhingra V. A case of extracranial metastasis of glioblastoma multiforme seen on bone scintigraphy. Mol Imaging Radionucl Ther. 2022. 31: 246-9
18. Witthayanuwat S, Pesee M, Supaadirek C, Supakalin N, Thamronganantasakul K, Krusun S. Survival analysis of glioblastoma multiforme. Asian Pac J Cancer Prev. 2018. 19: 2613-7
19. Wu W, Klockow J, Zhang M, Lafortune F, Chang E, Jin L. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021. 171: 105780
20. Zaki M, Mashouf L, Woodward E, Langat P, Gupta S, Dunn I. Genomic landscape of gliosarcoma: Distinguishing features and targetable alterations. Sci Rep. 2021. 11: 18009
21. Zhang W, Cai Y, Wang X, Wang X, Li Y, Han G. Bone metastases of glioblastoma: A case report and review of the literature. Front Oncol. 2021. 11: 705455