- Department of Surgery, Section of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
- Medical School, Aga Khan University Hospital, Karachi, Pakistan,
- Duke University School of Medicine, Durham, North Carolina, United States,
- Department of Pathology, Aga Khan University Hospital, Karachi, Pakistan.
Correspondence Address:
S. Ather Enam, Department of Surgery, Section of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_491_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Shakir1, Ahmed Altaf1, Hawra Hussain2, Syed Muhammad Aqeel Abidi2, Zoey Petitt3, Mahnoor Tariq1, Ahmed Gilani4, S. Ather Enam1. Unveiling the potential application of intraoperative brain smear for brain tumor diagnosis in low-middle-income countries: A comprehensive systematic review. 08-Sep-2023;14:325
How to cite this URL: Muhammad Shakir1, Ahmed Altaf1, Hawra Hussain2, Syed Muhammad Aqeel Abidi2, Zoey Petitt3, Mahnoor Tariq1, Ahmed Gilani4, S. Ather Enam1. Unveiling the potential application of intraoperative brain smear for brain tumor diagnosis in low-middle-income countries: A comprehensive systematic review. 08-Sep-2023;14:325. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12530
Abstract
Background: Immediate intraoperative histopathological examination of tumor tissue is indispensable for a neurosurgeon to track surgical resection. A brain smear is a simple, rapid, and cost-effective technique, particularly important in the diagnosis of brain tumors. The study aims to determine the effectiveness of intraoperative brain smear in the diagnosis of brain tumors in low- and middle-income countries (LMICs), while also evaluating its sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy.
Methods: A comprehensive search of the literature was conducted using PubMed, Scopus, and Google Scholar. The retrieved articles were independently screened by two reviewers. The data was extracted, processed, and organized using Microsoft Excel.
Results: A total of 59 out of 553 articles screened were included in the final analysis. The sensitivity and specificity of the intraoperative smear of brain tumors were found to be over 90% in most studies. The PPV was consistently above 90% in 11 studies, reaching 100% in one study and the NPV varied, ranging from 63% to 100%, and the accuracy was found to be >80% in most studies. One recurrent theme in the majority of the included studies was that an intraoperative brain smear is a cost-effective, quick, accessible, and accurate method of diagnosing brain tumors, requiring minimal training and infrastructure.
Conclusion: Intraoperative brain smear is a simple, rapid, cost-effective, and highly sensitive diagnostic modality for brain tumors. It can be a viable and accessible alternative to more traditional methods such as frozen sections and can be incorporated into neurosurgical practice in LMICs as a reliable and efficient diagnostic tool.
Keywords: Brain tumors, Intraoperative brain smear, Low- and middle-income countries
INTRODUCTION
Brain tumors are a group of malignancies that can originate from cells within the brain (primary tumors) or from systemic tumors that have spread to the brain (secondary tumors).[
For a neurosurgeon, an instant intraoperative histopathological examination of tumor tissue is a critical tool to monitor surgical resection by differentiating normal brain histology from a tumor.[
The process of obtaining a frozen section, the gold standard for intraoperative histopathological examination, is time-consuming and can delay surgical treatment. It involves delivering tissue to a laboratory, processing the specimen, preparing slides by technicians, and interpreting the slides by a pathologist.[
Since 1930, when Eisenhardt and Cushing proposed the use of a touch imprint for rapid tumor identification, cytological methods have been utilized to diagnose brain tumors.[
The main goals of intraoperative neuropathologic consultation for neurosurgeons are to guarantee that the diagnostic specimen was collected with the least amount of trauma and to ensure an immediate and appropriate treatment.[
Brain smear techniques are relatively cheap compared to other modalities available and can be conducted within the operating room without any specialized equipment or specialized technicians being involved, which holds advantages for low- and middle-income countries (LMICs).[
The objective of this study is to determine the usefulness of intraoperative brain smears in the diagnosis of brain tumors in LMICs through a systematic review of the existing literature. The review aims to evaluate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of intraoperative brain smears as a diagnostic tool in LMICs.
MATERIALS AND METHODS
This systematic review was conducted following our research protocol, which was based on the research question using population, intervention, control, and outcome. The preferred reporting items for systematic reviews and meta-analyses reporting standards were followed.[
Eligibility criteria
This review analyzes the use of intraoperative brain smears during surgical procedures of brain tumors, both primary and metastatic. Studies eligible for inclusion were observational studies, including cohort studies, and randomized and nonrandomized controlled trials. The literature searched included articles that presented epidemiological, clinical, and laboratory aspects of the use of brain smears during tumor procedures. All age groups were included in this review. Studies were excluded from the study: editorials, case reports, case series (n < 10), studies published as abstracts only, letters to the editor, book chapters, and theses, as well as articles on spine tumors or other brain pathologies.
Final histopathology served as the gold standard test for evaluating diagnostic accuracy. This provided a definitive diagnosis based on tissue examination and has been widely recognized for its reliability and validity. Only studies utilizing the final histopathology report as the reference standard were included in our analysis.
Search strategy
A comprehensive search was conducted across multiple electronic databases, including PubMed, Scopus, and Google Scholar, with no language restrictions. The search encompassed articles from inception to December 25, 2022, and all searches were performed on the same date. We searched for relevant phrases in the Medical Subject Headings database and selected the following free-text terms as keywords: “brain neoplasms” OR “brain tumor*” AND “brain smear” OR “intraoperative cytology” OR “intraoperative squash cytology” OR “imprint cytology” OR “touch cytology” to yield a comprehensive and inclusive dataset. The included studies were searched for forward citations to ensure that all the relevant literature are included in the study. The detailed search strategy for each database is available in the supplementary document.
Study selection
All retrieved studies’ titles and abstracts were initially screened by two reviewers (SAA and HH) for duplication and relevancy according to our research question. The final study selection was made after an independent review by two reviewers (SAA and HH) of the full texts of all possibly pertinent studies. A consensus was reached by a third author (MS) to settle conflicts.
Data extraction
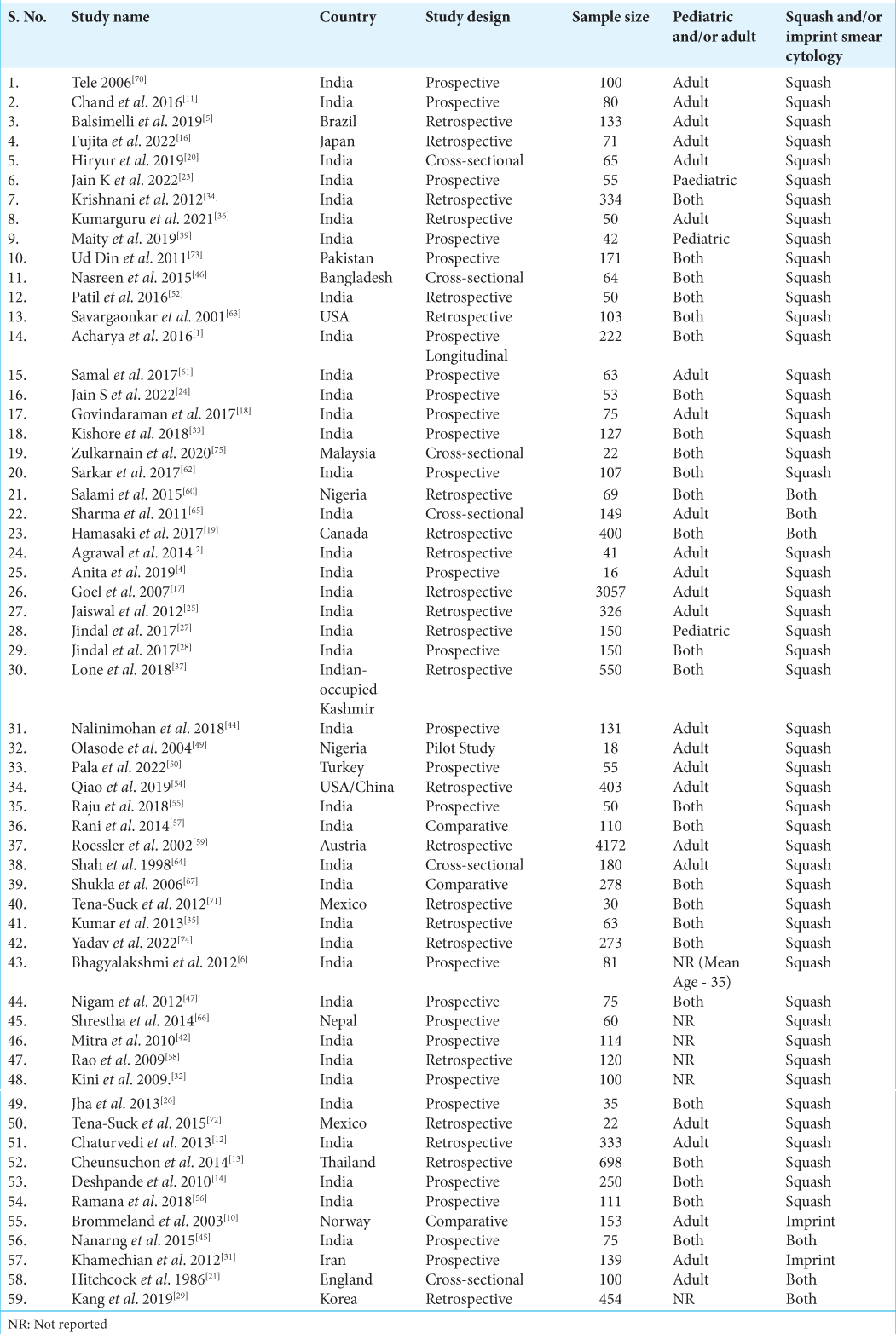
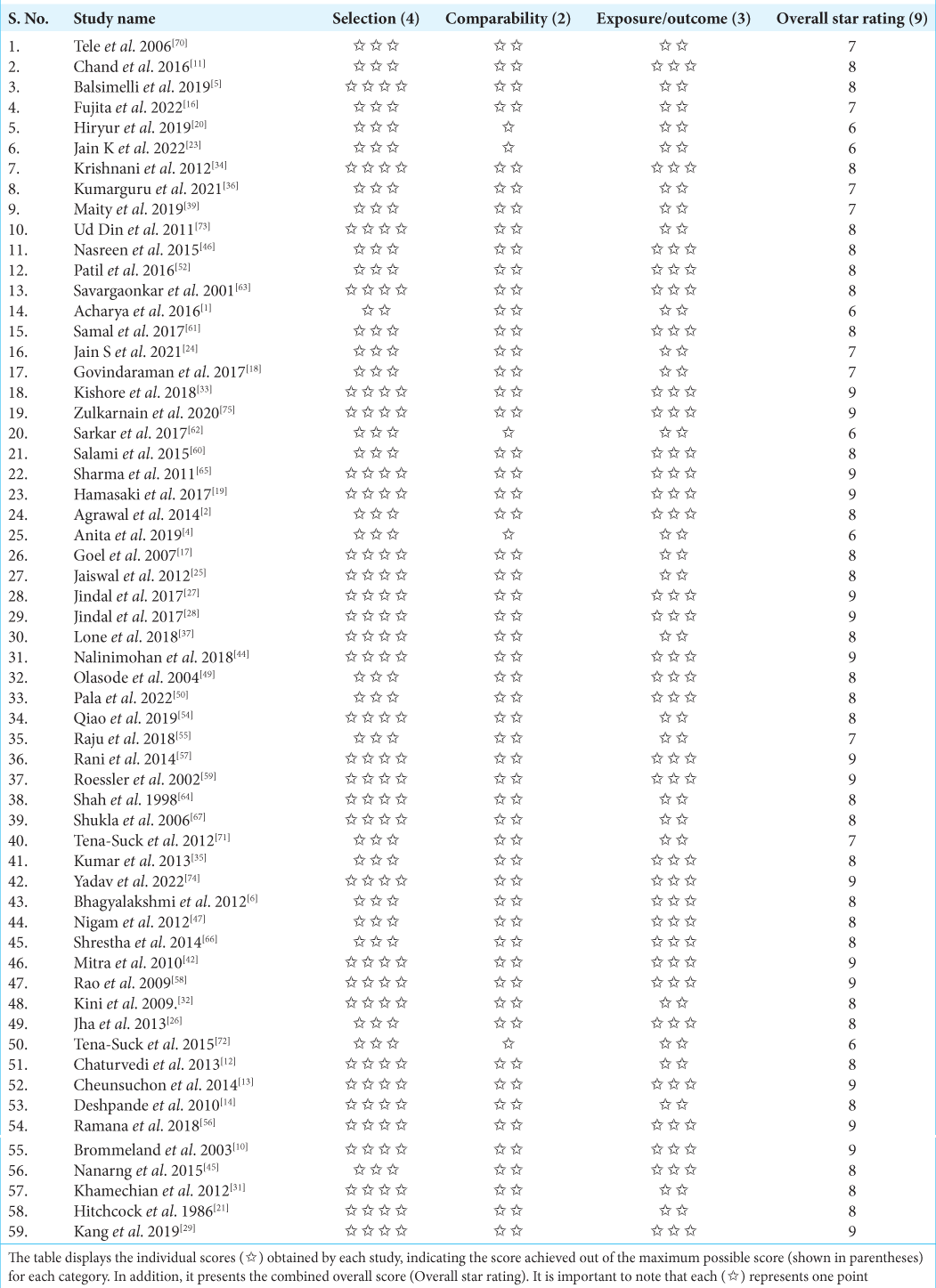
Study characteristics (study title, authors, date of publication, publication type, study location, and sample size), population characteristics, study objective, advantages of the mentioned brain smear techniques, test characteristics (accuracy, sensitivity, specificity, PPV, and NPV), and study outcomes were extracted from eligible articles. Two authors independently extracted the data (SAA, HH). A third author (MS) corrected errors in data extraction and double-checked the information collected. Two authors (SAA and AA) independently evaluated each study’s quality using the Newcastle–Ottawa scale quality assessment.[
The review underwent rigorous data validation, involving cross-referencing data from multiple sources, applying validation criteria, and resolving discrepancies through reviewer consensus. Data cleaning removed errors and inconsistencies while missing data were managed using the method of exclusion.
Data analysis
The data were processed and analyzed using Microsoft Excel. For ease of reporting and comprehension, the extracted data were cleaned and organized into tables.
RESULTS
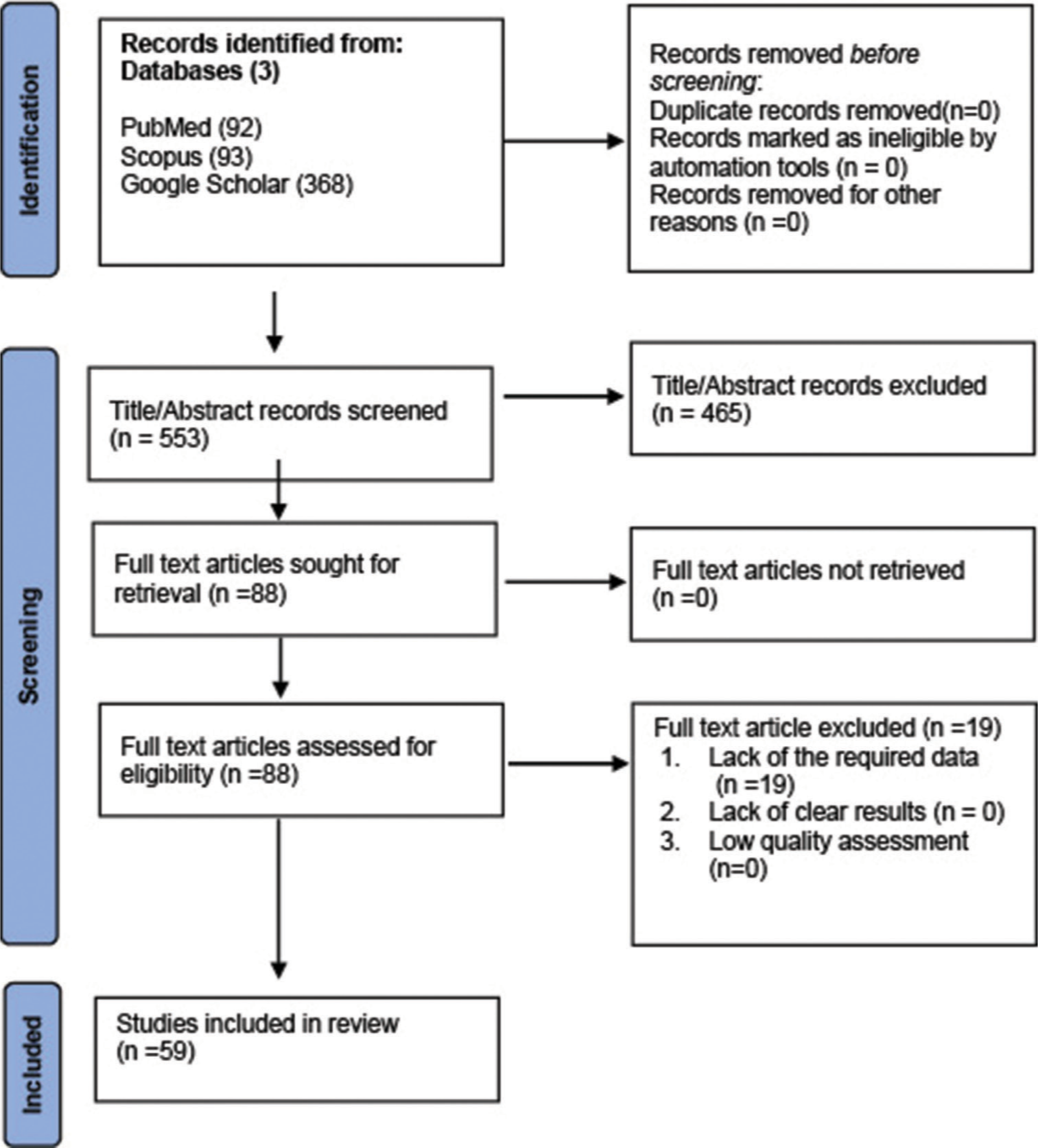
Our initial search for relevant studies identified a total of 553 articles. After removing duplicates and reviewing the titles and abstracts, 88 studies were reviewed in full text. A total of 59 articles were included in the final analysis.[
The process of screening and selecting these studies, including the removal of duplicates and review of titles and abstracts, is shown in
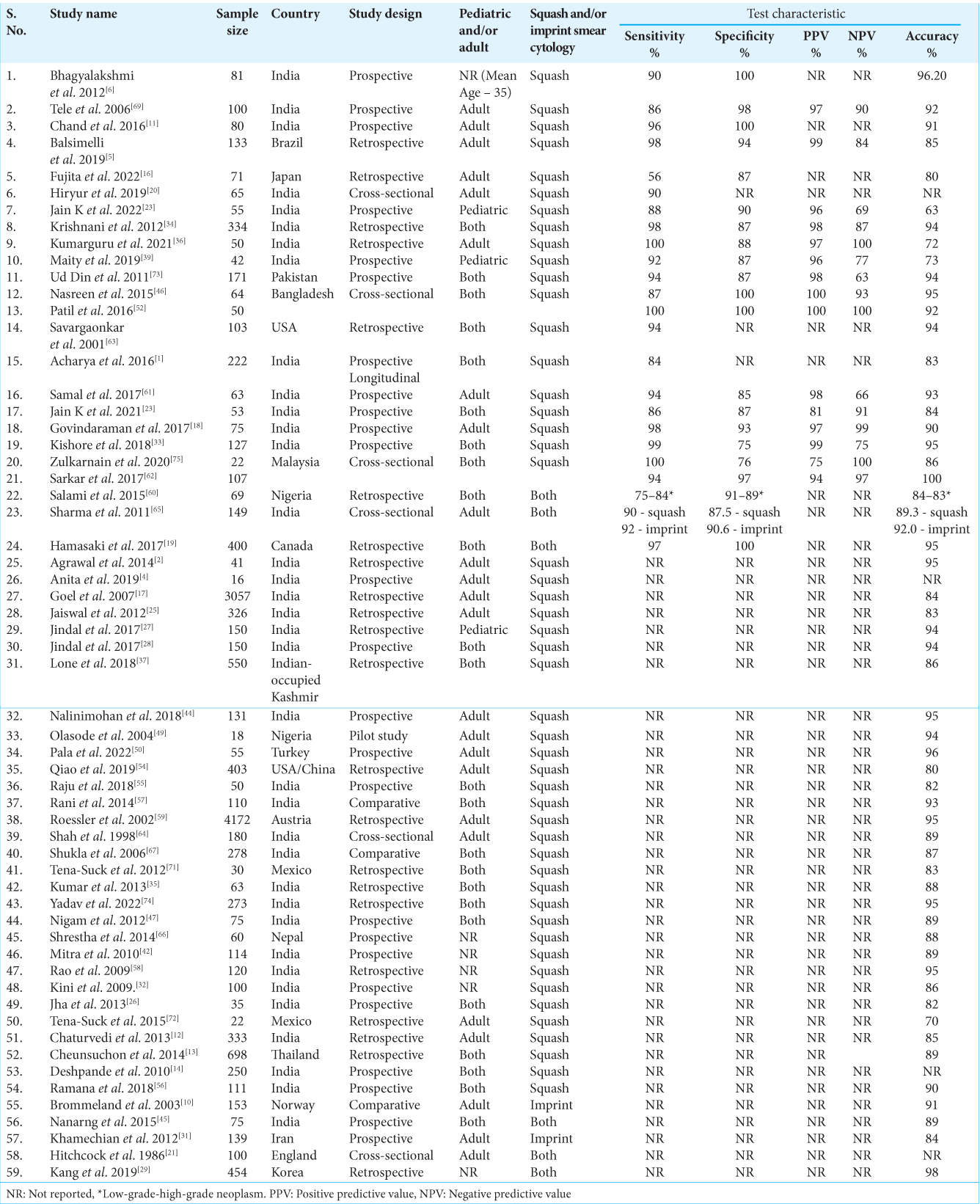
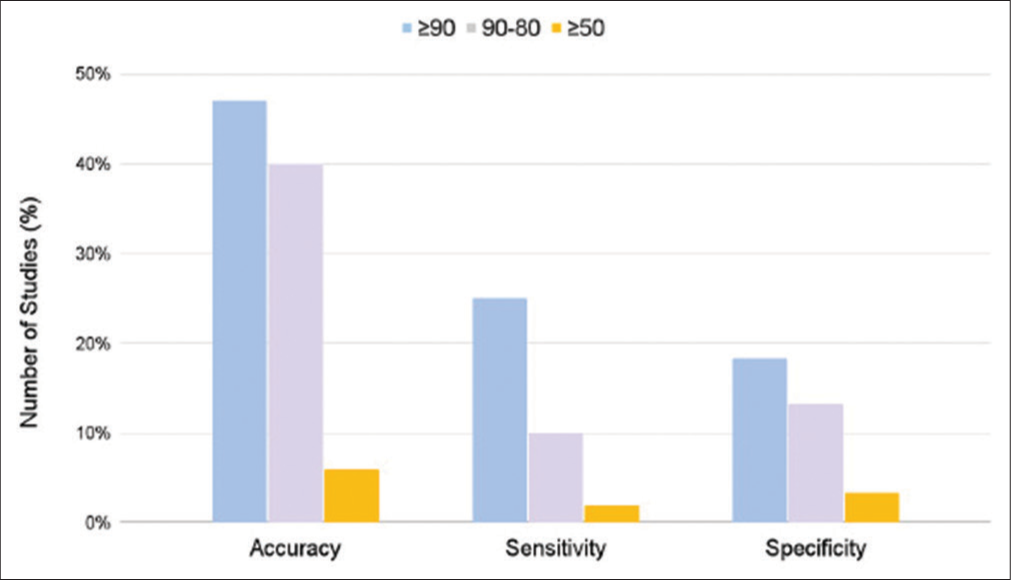
The results of the analysis indicate that the sensitivity of intraoperative smear in detecting brain tumors was found to be more than 90% in the majority of cases (12 studies) and 100% in two studies. However, it should be noted that one study reported a sensitivity of 56%. In terms of specificity, the findings showed greater variability, with six studies reporting a specificity of >90%, while one study reported a specificity of 100%. The lowest reported specificity was observed in the range of 75–76%.
The PPV was reported above 90% in the majority of the studies (11 studies) and in one study, it was 100%. However, the lowest value reported was 75%. The NPV demonstrated a wide range, with the lowest value reported being 63% and the highest being 100%, as documented in three studies. However, the majority of studies (seven in total) that reported NPV fell within the range of 80–100%.
Overall, the accuracy of intraoperative smears for detecting brain tumors was reported >80% in the majority of studies (47 studies). It is important to note that the accuracy of any diagnostic test can be influenced by various factors, including the specific technique used and the characteristics of the population being tested. The detailed results of the studies on sensitivity, specificity, PPV, NPV, and accuracy are shown in
Figure 2:
The Y-axis of the graph represents the frequency (%) of studies reporting the values of accuracy, sensitivity, and specificity of brain smear, while the X-axis represents the actual values of accuracy, sensitivity, and specificity. The values on the X-axis are categorized as ≥ 90, 90–80, and ≥ 50, which correspond to different levels of sensitivity, specificity, and accuracy.
Articles in this review also discussed numerous advantages of intraoperative smear including cost-effectiveness, minimal infrastructure requirements, rapid (10–15 min), accessibility, and accuracy in diagnosis. Intraoperative smear was reported to be relatively inexpensive compared to more complex tests, and it can be performed using basic equipment and facilities.
DISCUSSION
This is the first comprehensive review that evaluates the sensitivity, specificity, positive and NPVs, and accuracy of an intraoperative brain smear. It also highlights its advantages for resource-limited settings where there is a shortage of trained histopathologists and histopathology facilities.
This review reported the diagnostic accuracy of the intraoperative brain smear as >80%, as reported in the majority of studies. Sensitivity and specificity values were similarly high, with more than 90% reported in most studies. In addition, most studies reported positive and NPVs >90%. The main advantages reported include it being a rapid, simple, reliable, and cost-effective tool for diagnosis. Other advantages include high accuracy,[
A frequently cited benefit of intraoperative brain smears, as noted in many of the studies, is the considerably shorter time required for diagnosis compared to conventional techniques. As per the study by Jaiswal et al.,[
False positives and false negatives were reported in a few of the studies, as a small minority of the total sample size. One reason for this misdiagnosis through a squash smear was due to specific tumors such as ependymomas and meningiomas not being able to smear due to their firm nature.[
Intraoperative brain smears are highly accurate (95.3%) and reliable as a primary diagnostic tool for planning treatment.[
This review highlighted some of the limitations of squash smears in the diagnosis of certain brain tumors. One is the misrepresentation of tumor tissue due to absent histological artifacts, which leads to inaccurate diagnoses being made.[
This study is the first systematic review, to the best of our knowledge, conducted on the utility of intraoperative brain smears in brain tumor diagnosis. Overall, the results show that intraoperative brain smear is a rapid, simple, safe, cost-effective, and fairly accurate method of diagnosis of brain tumors which can improve service delivery efficiency and reduce the burden on healthcare systems in LMICs. This method could be especially beneficial in resource-limited settings, where the equipment for frozen sections is not often available and histopathological diagnosis also faces obstacles. There is a lack of trained histopathologists, limited availability of laboratory infrastructure, and limited advanced equipment to support the high demand for histopathological diagnosis. Our study reports the advantages of brain smear specifically for resource-limited settings, where other modalities of diagnosis are often not available.
Limitation
This systematic review has some limitations, which should be taken into consideration when interpreting the findings. The majority of the articles taken into consideration were observational studies and may not provide strong evidence for the diagnostic accuracy of brain smears. Most of the studies reviews were also conducted in LMICs, namely, India, so the results may not be applicable to other parts of the world.
Future direction
Further research is necessary to fully explore and understand the feasibility and implementation of intraoperative brain smears as a diagnostic modality for brain tumors in LMICs. Conducting further controlled studies, especially those that gather data from a larger range of countries, will provide better insight into the utility of the brain smear. Further, a comparison of the brain smear technique with other diagnostic modalities available will also provide valuable insight. Finally, guideline development regarding the use of brain smears in brain tumor diagnosis will be necessary for regulating the approach and improving the quality of care given worldwide.
CONCLUSION
Our systematic review revealed that intraoperative brain smear is a simple, rapid, cost-effective, and highly sensitive diagnostic modality for brain tumors in LMICs. It appears from the included literature that brain smears in settings with limited resources can be a viable and accessible alternative to more traditional methods such as frozen sections. Furthermore, the results of the studies reviewed suggest that brain smears could be incorporated into neurosurgical practice in LMICs as a reliable and efficient diagnostic tool.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SEARCH STRATEGY
Concept#1: (Intraoperative)
“Intraoperative Period”[Mesh] OR “Intraoperative Care”[Mesh]
Concept#2: (Brain Smear)
(“Brain Smear” OR “Intraoperative cytology” OR “Intraoperative squash cytology” OR “Intraoperative squash smear” OR “Squash Smear” OR “Imprint cytology” OR “touch cytology”)
Concept#3 (Brain Tumor)
“Brain Neoplasms”[Mesh] OR “Central Nervous System Neoplasms”[Mesh] OR “Brain tumor*” OR “brain cancer*” OR “CNS tumor*” OR “Nervous system tumor*” OR “NeuroOncology” OR “Intracranial neoplasm*” OR “Primary brain tumor*”
PubMed
((“Brain Neoplasms”[Mesh] OR “Central Nervous System Neoplasms”[Mesh] OR “Brain tumor*” OR “brain cancer*” OR “CNS tumor*” OR “Nervous system tumor*” OR “NeuroOncology” OR “Intracranial neoplasm*” OR “Primary brain tumor*”) AND (“Brain Smear” OR “Intraoperative cytology” OR “Intraoperative squash cytology” OR “Intraoperative squash smear” OR “Squash Smear” OR “Imprint cytology” OR “touch cytology”))
Scopus
((“Brain Neoplasms” OR “Central Nervous System Neoplasms” OR “Brain tumor*” OR “brain cancer*” OR “CNS tumor*” OR “Nervous system tumor*” OR “NeuroOncology” OR “Intracranial neoplasm*” OR “Primary brain tumor*”) AND (“Brain Smear” OR “Intraoperative cytology” OR “Intraoperative squash cytology” OR “Intraoperative squash smear” OR “Squash Smear” OR “Imprint cytology” OR “touch cytology”))
Google Scholar: (Using Publish or Perish)
“Brain Neoplasms” | “Central Nervous System Neoplasms” | “Brain tumor” | “Primary brain tumor” “Brain Smear” | “Intraoperative cytology” | “Intraoperative squash cytology” | “Intraoperative squash smear” | “Squash Smear” | “Imprint cytology” | “touch cytology”
References
1. Acharya S, Azad S, Kishore S, Kumar R, Arora P. Squash smear cytology, CNS lesions-Strengths and Limitations. Natl J Lab Med. 2016. 5: 1-7
2. Agrawal M, Chandrakar SK, Lokwani D, Purohit MR. Squash cytology in neurosurgical practice: A useful method in resource-limited setting with lack of frozen section facility. J Clin Diagn Res. 2014. 8: C09-12
3. Ahmad Z, Barakzai MA, Idrees R, Bhurgri Y. Correlation of intra-operative frozen section consultation with the final diagnosis at a referral center in Karachi, Pakistan. Indian J Pathol Microbiol. 2008. 51: 469-73
4. Anita AM, Bhargava N, Patil AG, Andola SK. Clicopathological correlation in metastatic central nervous system tumor. J Med Sci Clin Res. 2019. 7: 692-6
5. Balsimelli LB, Oliveira JC, Adorno FÁ, Brites CA, Bublitz GS, Tavares LC. Accuracy of intraoperative examination in central nervous system lesions: A study of 133 cases. Acta Cytol. 2019. 63: 224-32
6. Bhagyalakshmi A, Prasad KV, Uma P, Rao PS, Prasad PK, Hygreev RB. Role of squash smears, imaging and histopathology in diagnosing CNS lesions-a prospective study. Natl J Basic Med Sci. 2012. 2: 213-20
7. Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’Yasova D. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008. 113: 1953-68
8. Boopathy T, Karthika V. An analysis of CNS tumors in squash preparations with histological correlation. IOSR J Dent Med Sci. 2017. 16: 81-6
9. Brenner H, Kliebsch U. Dependence of weighted kappa coefficients on the number of categories. Epidemiology. 1996. 7: 199-202
10. Brommeland T, Lindal S, Straume B, Dahl IL, Hennig R. Does imprint cytology of brain tumours improve intraoperative diagnoses?. Acta Neurol Scand. 2003. 108: 153-6
11. Chand P, Amit S, Gupta R, Agarwal A. Errors, limitations, and pitfalls in the diagnosis of central and peripheral nervous system lesions in intraoperative cytology and frozen sections. J Cytol. 2016. 33: 93-7
12. Chaturvedi S, Pant I, Dua R, Gupta S. Analyzing agreement patterns of intraoperative central nervous system lesion reporting according to type and grade. J Cytol. 2013. 30: 179-84
13. Cheunsuchon P. Accuracy of intraoperative consultation of central nervous system lesions in Siriraj hospital. Siriraj Med J. 2014. 66: 113-9
14. Deshpande K, Surase S, Shedge R, D’costa G, Bharambe B. Accuracy and diagnostic yield of intraoperative squash smear technique in the rapid diagnosis of CNS lesions. Bombay Hosp J. 2010. 52: 153-60
15. Eisenhardt L, Cushing H. Diagnosis of intracranial tumors by supravital technique. Am J Pathol. 1930. 6: 541-552.7
16. Fujita H, Tajiri T, Machida T, Nomura N, Toguchi S, Itoh H. Scoring system for intraoperative diagnosis of intracranial schwannoma by squash cytology. Cytopathology. 2022. 33: 196-205
17. Goel D, Sundaram C, Paul TR, Uppin SG, Prayaga AK, Panigrahi MK. Intraoperative cytology (squash smear) in neurosurgical practice-pitfalls in diagnosis experience based on 3057 samples from a single institution. Cytopathology. 2007. 18: 300-8
18. Govindaraman P, Arumugam N, Ramasamy C, Prakasam G. Role of squash smear in intraoperative consultation of central nervous system tumors. J Sci Soc. 2017. 44: 7
19. Hamasaki M, Chang KH, Nabeshima K, Tauchi-Nishi PS. Intraoperative squash and touch preparation cytology of brain lesions stained with H+E and Diff-Quik™: A 20-year retrospective analysis and comparative literature review. Acta Cytol. 2018. 62: 44-53
20. Hiryur SP, Patel U, Sivaranjini N. Comparative study of frozen section and squash preparation for intraoperative diagnosis of CNS lesions and their correlation with paraffin sections in a tertiary care hospital, Vadodara. Int J Clin Diagn Pathol. 2019. 2: 263-7
21. Hitchcock E, Morris CS, Sotelo MG, Salmon M. Comparison of Smear and imprint techniques for rapid diagnosis in neurooncology. Surg Neurol. 1986. 26: 176-82
22. Jaafar H. Intra-operative frozen section consultation: Concepts, applications and limitations. Malays J Med Sci. 2006. 13: 4-12
23. Jain K, Sengupta M, Maity P, Chatterjee U, Chaudhuri S, Rajyalakshmi E. Comparison of frozen section and squash cytology as intra-operative diagnostic tool in pediatric CNS tumors. Neurol India. 2022. 70: 714-20
24. Jain S, Kaushal M, Choudhary A, Bhardwaj M. Comparative evaluation of squash smear and frozen section in the intraoperative diagnosis of central nervous system tumours. Cytopathology. 2022. 33: 107-13
25. Jaiswal S, Vij M, Jaiswal AK, Behari S. Intraoperative squash cytology of central nervous system lesions: A single center study of 326 cases. Diagn Cytopathol. 2012. 40: 104-12
26. Jha B, Patel V, Patel K, Agarwal A. Squash smear technique in intraoperative diagnosis of CNS tumors 889. Int J Med Sci Public Health. 2013. 4: 889-92
27. Jindal A, Diwan H, Kaur K, Sinha VD. Intraoperative squash smear in central nervous system tumors and its correlation with histopathology: 1 year study at a tertiary care centre. J Neurosci Rural Pract. 2017. 8: 221-4
28. Jindal A, Kaur K, Mathur K, Kumari V, Diwan H. Intraoperative squash smear cytology in CNS lesions: A study of 150 pediatric cases. J Cytol. 2017. 34: 217-20
29. Kang M, Chung DH, Kim NR, Cho HY, Ha SY, Lee S. Intraoperative frozen cytology of central nervous system neoplasms: An ancillary tool for frozen diagnosis. J Pathol Transl Med. 2019. 53: 104-11
30. Karnam S, Girish HC, Murgod S, Nayak VN, Varsha VK, Yanduri S. Rapid tissue processing technique: A novel method using methyl salicylate. J Oral Maxillofac Pathol. 2018. 22: 443
31. Khamechian T, Alizargar J, Mazoochi T. The value of touch preparation for rapid diagnosis of brain tumors as an intraoperative consultation. Iran J Med Sci. 2012. 37: 105-11
32. Kini JR, Jeyraj V, Jayaprakash CS, Indira S, Naik CN. Intraoperative consultation and smear cytology in the diagnosis of brain tumours. Kathmandu Univ Med J (KUMJ). 2008. 6: 453-7
33. Kishore S, Thakur B, Bhardwaj A, Kusum A. Diagnostic accuracy of squash cytology and role of GFAP immuno expression in glial tumors. Indian J Pathol Oncol. 2020. 5: 12-7
34. Krishnani N, Kumari N, Behari S, Rana C, Gupta P. Intraoperative squash cytology: Accuracy and impact on immediate surgical management of central nervous system tumours. Cytopathology. 2012. 23: 308-14
35. Kumar Verma S, Kumar R, Srivani J, Jonathan J. Diagnostic accuracy of squash preparations in central nervous system tumors. Iran J Pathol. 2013. 8: 227-34
36. Kumarguru BN, Santhipriya G, Kumar SK, Kumar RR, Ramaswamy AS, Janakiraman P. A comparative study of squash smear cytology diagnosis and radiological diagnosis with histopathology in central nervous system lesions. J Cytol. 2022. 39: 1-8
37. Lone MI, Jeelani T, Rashid G, Bashir N, Angmo D. Intraoperative squash cytology and histopathological correlation of primary temporal lobe lesions: A 6 year study at tertiary care centre of Kashmir, India. Int J Res Med Sci. 2018. 6: 2148-52
38. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
39. Maity P, Sengupta M, Jain K, Chaudhuri S, Chatterjee U, Datta C. Utility of intraoperative squash cytology in diagnosis of paediatric central nervous system lesions. Diagn Cytopathol. 2019. 47: 428-33
40. Marshall LF, Adams H, Doyle D, Graham DI. The histological accuracy of the smear technique for neurosurgical biopsies. J Neurosurg. 1973. 39: 82-8
41. McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA. 2018. 319: 388-96
42. Mitra S, Kumar M, Sharma V, Mukhopadhyay D. Squash preparation: A reliable diagnostic tool in the intraoperative diagnosis of central nervous system tumors. J Cytol. 2010. 27: 81-5
43. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009. 339: 332-6
44. Nalinimohan C, Padmaja K, Shailaja P, Deshpande AK. Intraoperative squash cytology of central nervous system lesions: review of an eight-year experience. Indian J Pathol Res Pract. 2018. 7: 1095-8
45. Nanarng V, Jacob S, Mahapatra D, Mathew JE. Intraoperative diagnosis of central nervous system lesions: Comparison of squash smear, touch imprint, and frozen section. J Cytol. 2015. 32: 153-8
46. Nasreen S, Shahab M, Ahamad U, Rahman N. Intraoperative diagnosis of Cns tumor by crush cytologic technique and its histopathological correlation. J Chittagong Med Coll Teach Assoc. 2015. 26: 40-3
47. Nigam SK, Nigam N, Mishra A. Diagnostic accuracy of squash Smear technique in brain tumors. J Evol Med Dent Sci. 2012. 1: 538-45
48. Novis DA, Zarbo RJ. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 3286.8 frozen sections in 700 hospitals. Arch Pathol Lab Med. 1997. 121: 559-67
49. Olasode BJ, Ironside JW. The brain smear, a rapid affordable intraoperative diagnostic technique for brain tumours appropriate for Africa. Trop Doct. 2004. 34: 223-5
50. Pala EE, Doğan E, Ekmekçi S, Özamrak BG, Çamlar M Pala. Diagnostic value of smears and frozen sections in neuropathology practice diagnostic value of smears and frozen sections in neuropathology practice: Institutional Experience. J Tepecik Educ Res Hosp. 2022. 32: 51-8
51. Patel AP, Fisher JL, Nichols E, Abd-Allah F, Abdela J, Abdelalim A. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. 18: 376-93
52. Patil SS, Kudrimoti JK, Agarwal RD, Jadhav MV, Chuge A. Utility of squash smear cytology in intraoperative diagnosis of central nervous system tumors. J Cytol. 2016. 33: 205-9
53. Plesec TP, Prayson RA. Frozen section discrepancy in the evaluation of nonneoplastic central nervous system samples. Ann Diagn Pathol. 2009. 13: 359-66
54. Qiao N, Swearingen B, Hedley-Whyte ET, Tritos NA. The utility of intraoperative cytological smear and frozen section in the surgical management of patients with Cushing’s disease due to pituitary microadenomas. Endocr Pathol. 2019. 30: 180-8
55. Raju VS, Srirangaramasamy J, Balasubramanian D, Balamurugan M, Rajesh H, Murugan N. Diagnostic accuracy of squash cytology for rapid intraoperative diagnosis in tumors of nervous system. IOSR J Dent Med Sci. 2018. 17: 27-32
56. Ramana PV, Shyamala S. Diagnostic accuracy of central nervous system tumors by squash cytology. Int Arch Integr Med. 2018. 5: 29-35
57. Rani H, Kulkarni P, Dinesh US, Rao RV, Melkundi S. Comparison of squash smears and frozen sections versus paraffin sections in the intra-operative diagnosis of central nervous system lesions. El Med J. 2014. 2: 101
58. Rao S, Rajkumar A, Ehtesham MD, Duvuru P. Challenges in neurosurgical intraoperative consultation. Neurol India. 2009. 57: 464-8
59. Roessler K, Dietrich W, Kitz K. High diagnostic accuracy of cytologic smears of central nervous system tumors. A 15-year experience based on 4.172 patients. Acta Cytol. 2002. 46: 667-74
60. Salami A, Azeez A, Malomo A, Oluwasola A, Adeleye A, Ogun G. Correlation of intraoperative cytological and final histological diagnoses: A retrospective 10-year study of neurosurgical cases from Ibadan, Nigeria. Diagn Cytopathol. 2015. 43: 195-201
61. Samal S, Kalra R, Sharma J, Singh I, Panda D, Ralli M. Comparison between crush/squash cytology and frozen section preparation in intraoperative diagnosis of central nervous system lesions. Oncol J India. 2017. 1: 25
62. Sarkar S, Sengupta M, Datta C, Chatterjee U, Ghosh SN. Evaluation of intraoperative cytological smears for diagnosis of brain tumors with special reference to immunohistochemistry. Indian J Med Paediatr Oncol. 2017. 38: 296-301
63. Savargaonkar P, Farmer PM. Utility of intra-operative consultations for the diagnosis of central nervous system lesions. Ann Clin Lab Sci. 2001. 31: 133-9
64. Shah AB, Muzumdar GA, Chitale AR, Bhagwati SN. Squash preparation and frozen section in intraoperative diagnosis of central nervous system tumors. Acta Cytol. 1998. 42: 1149-54
65. Sharma N, Misra V, Singh PA, Gupta SK, Debnath S, Nautiya A. Comparative efficacy of imprint and squash cytology in diagnosing lesions of the central nervous system. Asian Pac J Cancer Prev. 2011. 12: 1693-6
66. Shrestha S, Thapa BK, Bhattarai B. Smear technique for intraoperative diagnosis of central nervous system neoplasms. J Pathol Nepal. 2014. 4: 544-7
67. Shukla K, Parikh B, Shukla J, Trivedi P, Shah B. Accuracy of cytologic diagnosis of central nervous system tumours in crush preparation. Indian J Pathol Microbiol. 2006. 49: 483-6
68. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010. 25: 603-5
69. Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A. Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015. 16: 1193-224
70. Tele F, editors. An Analysis of CNS Tumors in Squash Preparations with Histological Correlation. 2006. p.
71. Tena-Suck M, Salinas-Lara C, Vega-Orozco R, RembaoBojorquez D, Gelista N. Crush intraoperatory analysis in craniopharyngioma. Diagn Cytopathol. 2012. 40: 865-70
72. Tena-Suck ML, Estrada-Natoli L, Corona-Cobian LE, TorralRizo VH. Chordomas; crush intraoperative analysis. J Cytol Histol. 2015. 6: 3
73. Ud Din N, Memon A, Idress R, Ahmad Z, Hasan S. Central nervous system lesions: Correlation of intraoperative and final diagnoses, six year experience at a referral centre in a developing country, Pakistan. Asian Pac J Cancer Prev. 2011. 12: 1435-7
74. Yadav M, Sharma P, Singh V, Tewari R, Mishra PS, Roy K. An audit of diagnostic disparity between intraoperative frozen section diagnosis and final histopathological diagnosis of central nervous system lesions at a tertiary care center. J Lab Physicians. 2022. 14: 384-93
75. Zulkarnain S, Yunus N, Kandasamy R, Zun AB, Mat Zin AA. Evaluation study of intraoperative cytology smear and frozen section of glioma. Asian Pac J Cancer Prev. 2020. 21: 3085-91