- Department of Surgery, Medical College, King Faisal University, Hofuf, Saudi Arabia
- College of Medicine, King Faisal University, Hofuf, Saudi Arabia
- Department of Neurosurgery, King Fahad Hospital, Hofuf, Saudi Arabia
- Research Center, Almoosa Specialist Hospital, Almoosa College of Health Sciences, Al Mubarraz, Saudi Arabia
Correspondence Address:
Mohmmed Saud AlShammri, College of Medicine, King Faisal University, Hofuf, Saudi Arabia.
DOI:10.25259/SNI_926_2024
Copyright: © 2025 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulsalam Mohammed Aleid1, Mohmmed Saud AlShammri2, Saud Nayef Aldanyowi1, Awn Abdulmohsen Alessa3, Abdulmonem Ali Alhussain3, Abbas Al Mutair4. Use of repetitive transcranial magnetic stimulation in traumatic brain injury: A systematic review and meta-analysis of randomized controlled trials. 09-May-2025;16:175
How to cite this URL: Abdulsalam Mohammed Aleid1, Mohmmed Saud AlShammri2, Saud Nayef Aldanyowi1, Awn Abdulmohsen Alessa3, Abdulmonem Ali Alhussain3, Abbas Al Mutair4. Use of repetitive transcranial magnetic stimulation in traumatic brain injury: A systematic review and meta-analysis of randomized controlled trials. 09-May-2025;16:175. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13544
Abstract
Background: Traumatic brain injury (TBI) is an injury resulting from external force exerted directly or indirectly on the skull. This is presently the major cause of mortality and disability among youth globally. Repetitive transcranial magnetic stimulation (rTMS) was proposed for the treatment of various neurological disorders such as TBI. We conducted the current systematic review and meta-analysis to investigate the efficacy of rTMS in TBI patients.
Methods: We conducted our database searching on PubMed, Scopus, and Web of Science from inception till August 2024 to look for articles that fulfil our aim. The search strategy was based on two main keywords: “Transcranial magnetic stimulation” AND “Traumatic brain injury.” We conducted the pooled analysis of continuous variables using standardized mean difference (SMD) due to difference in measurement scales.
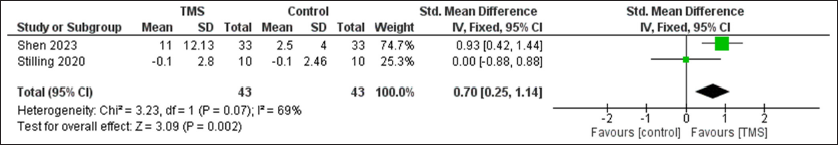
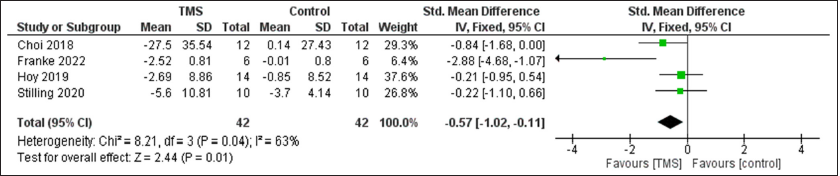
Results: Seven randomized controlled trials were included. A statistically significant improvement in cognitive function was observed after rTMS compared to control group with SMD of 0.7 (95% confidence interval [CI]: 0.25, 1.14, P = 0.002) with non-significant heterogeneity, and pain with SMD of −0.57 (95% CI: −1.02, −0.11, P = 0.01), I2 = 64%, P = 0.04. However, no difference was observed between the two groups regarding depression with SMD of −0.1 (95% CI: −0.54, 0.35, P = 0.67).
Conclusion: The use of rTMS is associated with improved cognitive functions and reduction in pain. No effect was observed regarding depression but future studies are still warranted in this important clinical field.
Keywords: Cognition, Depression, Pain, Repetitive transcranial magnetic stimulation, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is an injury resulting from external force exerted directly or indirectly on the skull. This is presently the major cause of mortality and disability among youth globally.[
Chronic pain commonly occurs in patients with moderate TBI, with prevalence reported as high as 75%.[
Transcranial magnetic stimulation (TMS) is an Food and Drug Administration-approved therapy for serious depression that is resistant to medicine in the United States.[
Furthermore, a prior study indicated that the treatment’s placement might contribute to alleviating post-concussive symptoms.[
MATERIALS AND METHODS
We used the guidelines of Cochrane handbook for systematic reviews and meta-analysis in addition to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to conduct this study.[
Database searching
We conducted our database searching on PubMed, Scopus, and Web of Science from inception till August 2024 to look for articles that fulfil our aim. The search strategy was based on two main keywords: “Transcranial magnetic stimulation” AND “Traumatic brain injury.” The resulting articles were gathered together and uploaded to Rayyan.[
Eligibility criteria and screening
We included articles which were randomized controlled trials (RCTs) investigating the use of rTMS in TBI patients. We excluded reviews, observational studies, and case reports. We conducted the title and abstract screening to find whether the articles matched our criteria or not. This process was followed by full-text screening to ensure that the included articles from the previous step were eligible for inclusion.
Data extraction and outcome measures
We extracted the baseline data of the included studies including study ID, groups, sample size, age, gender, and comorbidities of the included patients. Regarding the outcomes, we extracted the following: pain scales at baseline and after treatment including numeric pain rating scale, McGill Pain, Debilitating headache exacerbation composite score, and Headache Impact Test-6, depression including Hamilton Depression Rating Scale, Patient Health Questionnaire-9, and Montgomery-Asberg Depression Rating Scale, and cognition using coma recovery scale revised, and Montreal Cognitive Assessment.
Risk of bias assessment
This process was conducted using the Cochrane’s risk of bias assessment 2 tool (Rob-2).[
Statistical analysis
All the statistical procedures were done using Review Manager software version 5.4.[
RESULTS
Screening results
After searching the databases, the search strategy yielded a total of 199 articles. We removed 77 duplicates and conducted title and abstract screening for 122 articles. We excluded a total of 112 articles and conducted full-text screening for the remaining 10 articles to include 7 of them in the meta-analysis.[
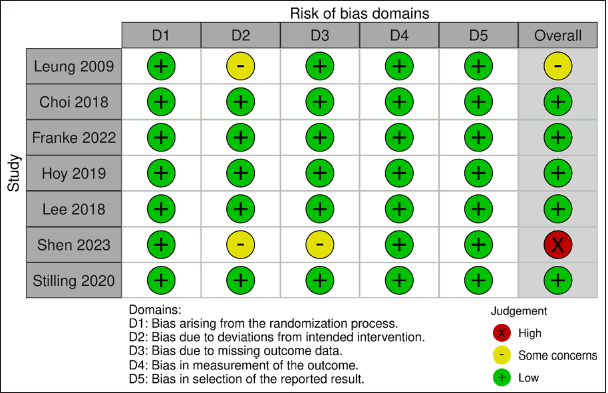
Risk of bias assessment
According to Rob-2, five studies had low risk of bias, one had high risk, and one had some concerns [
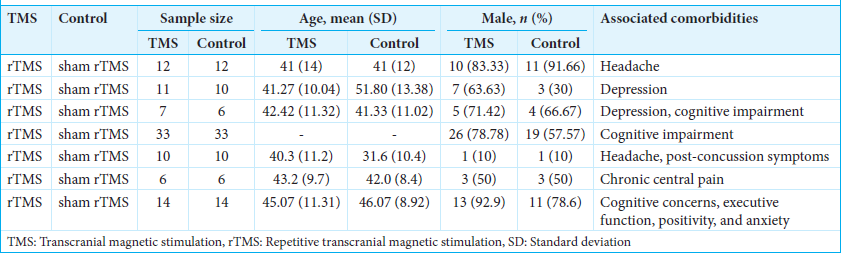
Baseline characteristics
We included a total of seven RCTs comparing rTMS with sham rTMS. Some comorbidities were reported such as headache, depression, and cognitive impairment [
Statistical analysis
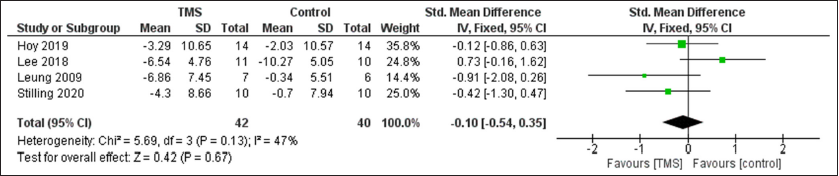
A statistically significant improvement in cognitive function was observed after TMS compared to control group with SMD of 0.7 (95% CI: 0.25, 1.14, P = 0.002) with non-significant heterogeneity, and pain with SMD of −0.57 (95% CI: −1.02, −0.11, P = 0.01), I2 = 64%, P = 0.04. However, no difference was observed between the two groups regarding depression with SMD of −0.1 (95%CI: −0.54, 0.35, P = 0.67) [
DISCUSSION
Main findings
The current systematic review and meta-analysis aimed to demonstrate the effectiveness of TMS in TBI patients. TMS was observed to be effective regarding the reduction in pain and increase in cognition. However, no significant difference was observed between patients who received TMS and those who did not receive regarding the effect on depression.
Cognition
The manifestation of consciousness disturbance following head trauma is associated with ischemic-hypoxic necrosis of cerebral tissue. Cerebral ischemia and hypoxia directly induce the cessation of function in specific brain regions; for example, the cerebral cortex fails to activate efficiently, resulting in an imbalance within the neural network associated with consciousness. Cognition and awakening are often regarded as correlated with consciousness. Hinter-Buchner designates these two as the constituents of awareness and the switching mechanism. The content of consciousness pertains to the advanced functions of the cerebral cortex, encompassing behavioral responses such as memory, cognition, orientation, motor skills, speech, and audiovisual processing. The mechanisms regulating consciousness can stimulate the cerebral cortex, sustain arousal, and preserve wakefulness. Consequently, the efficient activation of the cerebral cortex and the regulation of the brain–brain functional network are crucial for the emergence of disorders of consciousness. High-frequency rTMS targets the afflicted cerebral hemisphere and directly enhances its excitability.[
Furthermore, some perspectives suggest that rTMS alters cortical metabolism and cerebral blood flow by modulating the excitability of the local cerebral cortex, influencing neurotransmitters and their transmission, enhancing the reversibility of damaged cells, and facilitating the recovery of brain function.[
Depression
In a prior study, EEG data taken a median of 4 h post-rTMS stimulation revealed a power increase in delta, theta, and alpha waves, which associated with clinical improvement in depression.[
Pain
rTMS may serve as an advantageous therapeutic intervention for the management of chronic central pain resulting from moderate TBI and for enhancing quality of life. In 2006, Hirayama et al. enrolled 20 patients suffering from intractable neuropathic pain and performed high-frequency rTMS stimulation on the primary motor cortex M1, postcentral gyrus, premotor region, and supplementary motor area. They determined that M1 is the exclusive target capable of alleviating neuropathic pain.[
The present systematic review and meta-analysis is limited by the variability in measurement scales and the small sample size in most of the outcomes. We recommend future large size RCTs to validate our findings.
CONCLUSION
The use of rTMS is associated with improved cognitive functions and reduction in pain. No effect was observed regarding depression but future studies are still warranted in this important clinical field.
Authors’ contributions:
All authors substantially contributed to the study, including drafting the manuscript, conducting literature searches, analyzing data, critically reviewing the manuscript, and approving the final version for publication.
Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship:
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. KFU251297].
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abelson-Mitchell N. Epidemiology and prevention of head injuries: Literature review. J Clin Nurs. 2008. 17: 46-57
2. Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: A neuroanatomical review. Brain Res. 2004. 1000: 40-56
3. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J Mol Neurosci. 2008. 34: 51-61
4. Bakker N, Shahab S, Giacobbe P, Blumberger DM, Daskalakis ZJ, Kennedy SH. rTMS of the dorsomedial prefrontal cortex for major depression: Safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015. 8: 208-15
5. Bernat JL. Chronic disorders of consciousness. Lancet (London England). 2006. 367: 1181-92
6. Boivie J, Leijon G, Johansson I. Central post-stroke pain--a study of the mechanisms through analyses of the sensory abnormalities. Pain. 1989. 37: 173-85
7. Brunelin J, Poulet E, Boeuve C, Zeroug-vial H, d’Amato T, Saoud M. Efficacité de la stimulation magnétique transcrânienne (rTMS) dans le traitement de la dépression: Revue de la littérature [Efficacy of repetitive transcranial magnetic stimulation (rTMS) in major depression: A review]. L’Encephale. 2007. 33: 126-34
8. Cavanagh JF. Cortical delta activity reflects reward prediction error and related behavioral adjustments, but at different times. NeuroImage. 2015. 110: 205-16
9. Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control. 2009. 13: 442-53
10. Choi GS, Kwak SG, Lee HD, Chang MC. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: A pilot study. J Rehabil Med. 2018. 50: 246-52
11. De Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: The role of endogenous opioids. Pain. 2011. 152: 320-6
12. De Pascalis V, Scacchia P. The influence of reward sensitivity, heart rate dynamics and EEG-delta activity on placebo analgesia. Behav Brain Res. 2019. 359: 320-32
13. Devulder J, Crombez E, Mortier E. Central pain: An overview. Acta Neurol Belg. 2002. 102: 97-103
14. Emara TH, Moustafa RR, ElNahas NM, ElGanzoury AM, Abdo TA, Mohamed SA. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010. 17: 1203-9
15. Franke LM, Gitchel GT, Perera RA, Hadimani RL, Holloway KL, Walker WC. Randomized trial of rTMS in traumatic brain injury: Improved subjective neurobehavioral symptoms and increases in EEG delta activity. Brain Inj. 2022. 36: 683-92
16. García-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D. Electrical stimulation of motor cortex for pain control: A combined PET-scan and electrophysiological study. Pain. 1999. 83: 259-73
17. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013. 26: 13-8
18. Giacino JT, Trott CT. Rehabilitative management of patients with disorders of consciousness: Grand rounds. J Head Trauma Rehabil. 2004. 19: 254-65
19. Harmony T. The functional significance of delta oscillations in cognitive processing. Front Integr Neurosci. 2013. 7: 83
20. Hasan M, Whiteley J, Bresnahan R, MacIver K, Sacco P, Das K. Somatosensory change and pain relief induced by repetitive transcranial magnetic stimulation in patients with central poststroke pain. Neuromodulation. 2014. 17: 731-6
21. Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M. Reduction of intractable deafferentation pain by navigation-guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain. 2006. 122: 22-7
22. Hong JH, Bai DS, Jeong JY, Choi BY, Chang CH, Kim SH. Injury of the spino-thalamo-cortical pathway is necessary for central post-stroke pain. Eur Neurol. 2010. 64: 163-8
23. Hosomi K, Kishima H, Oshino S, Hirata M, Tani N, Maruo T. Cortical excitability changes after high-frequency repetitive transcranial magnetic stimulation for central poststroke pain. Pain. 2013. 154: 1352-7
24. Hoy KE, McQueen S, Elliot D, Herring SE, Maller JJ, Fitzgerald PB. A Pilot investigation of repetitive transcranial magnetic stimulation for post-traumatic brain injury depression: Safety, tolerability, and efficacy. J Neurotrauma. 2019. 36: 2092-8
25. Iaccarino MA, Bhatnagar S, Zafonte R. Rehabilitation after traumatic brain injury. Handb Clin Neurol. 2015. 127: 411-22
26. Jang SH, Lee HD. Central pain due to spinothalamic tract injury caused by indirect head trauma following a pratfall. Brain Inj. 2016. 30: 933-6
27. Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg psychiatry. 2005. 76: 833-8
28. Kim JH, Ahn SH, Cho YW, Kim SH, Jang SH. The Relation between injury of the spinothalamocortical tract and central pain in chronic patients with mild traumatic brain injury. J Head Trauma Rehabil. 2015. 30: E40-6
29. Knight RG, Harnett M, Titov N. The effects of traumatic brain injury on the predicted and actual performance of a test of prospective remembering. Brain Inj. 2005. 19: 19-27
30. Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012. 36: 677-95
31. Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020. 27: 741-56
32. Koski L, Kolivakis T, Yu C, Chen JK, Delaney S, Ptito A. Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J Neurotrauma. 2015. 32: 38-44
33. Lapitska N, Gosseries O, Delvaux V, Overgaard M, Nielsen F, Maertens de Noordhout A. Transcranial magnetic stimulation in disorders of consciousness. Rev Neurosci. 2009. 20: 235-50
34. Lee SA, Kim MK. Effect of low frequency repetitive transcranial magnetic stimulation on depression and cognition of patients with traumatic brain injury: A randomized controlled trial. Med Sci Monit. 2018. 24: 8789-94
35. Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM. rTMS for suppressing neuropathic pain: A meta-analysis. J Pain. 2009. 10: 1205-16
36. Li J, Jiang JY. Chinese Head Trauma Data Bank: Effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012. 29: 96-100
37. Lipton RB, Pearlman SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics. 2010. 7: 204-12
38. Louise-Bender Pape T, Rosenow J, Lewis G, Ahmed G, Walker M, Guernon A. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul. 2009. 2: 22-35
39. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008. 7: 728-41
40. Manganotti P, Formaggio E, Storti SF, Fiaschi A, Battistin L, Tonin P. Effect of high-frequency repetitive transcranial magnetic stimulation on brain excitability in severely brain-injured patients in minimally conscious or vegetative state. Brain Stimul. 2013. 6: 913-21
41. Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: A randomized, placebo-controlled study. J Neurol. 2013. 260: 2793-801
42. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009. 339: b2535
43. Mylius V, Borckardt JJ, Lefaucheur JP. Noninvasive cortical modulation of experimental pain. Pain. 2012. 153: 1350-63
44. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: A systematic review. JAMA. 2008. 300: 711-9
45. Noda Y, Nakamura M, Saeki T, Inoue M, Iwanari H, Kasai K. Potentiation of quantitative electroencephalograms following prefrontal repetitive transcranial magnetic stimulation in patients with major depression. Neurosci Res. 2013. 77: 70-7
46. Noh JS, Lim JH, Choi TW, Jang SG, Pyun SB. Effects and safety of combined rTMS and action observation for recovery of function in the upper extremities in stroke patients: A randomized controlled trial. Restor Neurol Neurosci. 2019. 37: 219-30
47. Ofek H, Defrin R. The characteristics of chronic central pain after traumatic brain injury. Pain. 2007. 131: 330-40
48. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016. 5: 210
49. Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: Possible pathways for antinociception. Pain. 2012. 153: 2359-69
50. Piccione F, Cavinato M, Manganotti P, Formaggio E, Storti SF, Battistin L. Behavioral and neurophysiological effects of repetitive transcranial magnetic stimulation on the minimally conscious state: A case study. Neurorehabil Neural Repair. 2011. 25: 98-102
51. Potapov AA, Krylov VV, Gavrilov AG, Kravchuk AD, Likhterman LB, Petrikov SS. Guidelines for the management of severe traumatic brain injury. Part 3. Surgical management of severe traumatic brain injury (Options). Zh Vopr Neirokhir Im N N Burdenko. 2016. 80: 93-101
52. Review Manager (RevMan). Version (5.4) The Cochrane collaboration. Available from: https://www.revman.cochrane.org [Last accessed on 2025 Mar 13].
53. Saitoh Y, Osaki Y, Nishimura H, Hirano S, Kato A, Hashikawa K. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J Neurosurg. 2004. 100: 935-9
54. Shen L, Huang Y, Liao Y, Yin X, Huang Y, Ou J. Effect of high-frequency repetitive transcranial magnetic stimulation over M1 for consciousness recovery after traumatic brain injury. Brain Behav. 2023. 13: e2971
55. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019. 366: l4898
56. Stilling J, Paxman E, Mercier L, Gan LS, Wang M, Amoozegar F. Treatment of persistent post-traumatic headache and post-concussion symptoms using repetitive transcranial magnetic stimulation: A pilot, double-blind, randomized controlled trial. J Neurotrauma. 2020. 37: 312-23
57. Styrke J, Stålnacke BM, Sojka P, Björnstig U. Traumatic brain injuries in a well-defined population: Epidemiological aspects and severity. J Neurotrauma. 2007. 24: 1425-36
58. Sveen U, Guldager R, Soberg HL, Andreassen TA, Egerod I, Poulsen I. Rehabilitation interventions after traumatic brain injury: A scoping review. Disabil Rehabil. 2022. 44: 653-60
59. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R. A classification of chronic pain for ICD-11. Pain. 2015. 156: 1003-7
60. Tsai PY, Wang CP, Ko JS, Chung YM, Chang YW, Wang JX. The persistent and broadly modulating effect of inhibitory rTMS in nonfluent aphasic patients: A sham-controlled, double-blind study. Neurorehabil Neural Repair. 2014. 28: 779-87
61. Vestergaard K, Nielsen J, Andersen G, Ingeman-Nielsen M, Arendt-Nielsen L, Jensen TS. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. Pain. 1995. 61: 177-86