- Department of Neurosurgery, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
Correspondence Address:
Masashi Kuwabara, Department of Neurosurgery, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan.
DOI:10.25259/SNI_868_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masashi Kuwabara, Takahito Okazaki, Daizo Ishii, Hiroshi Kondo, Masahiro Hosogai, Takeshi Hara, Yuyo Maeda, Nobutaka Horie. Usefulness of combined bypass surgery for moyamoya disease in infants under 1 year of age: A technical case report. 01-Mar-2024;15:72
How to cite this URL: Masashi Kuwabara, Takahito Okazaki, Daizo Ishii, Hiroshi Kondo, Masahiro Hosogai, Takeshi Hara, Yuyo Maeda, Nobutaka Horie. Usefulness of combined bypass surgery for moyamoya disease in infants under 1 year of age: A technical case report. 01-Mar-2024;15:72. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12771
Abstract
Background: Among pediatric cases of moyamoya disease (MMD), cerebral ischemic symptoms often progress and worsen rapidly in infants under one year of age; therefore, it is important to treat them as early as possible. However, direct bypass surgery is often technically difficult for infants due to their small blood vessels. Here, we describe our technique to resolve the technical challenges encountered during superficial temporal artery-to-middle cerebral artery (STA-MCA) bypass surgery in infants aged
Case Description: We performed bilateral STA-MCA and indirect bypass in a 1-year-old girl with MMD and cerebral infarction. Before treatment, a peripherally inserted central venous catheter (PICC) was placed to avoid ischemic attacks associated with crying, dehydration, and malnutrition. All examinations and procedures that would be stressful to the patient, such as blood examinations, were performed using PICC or under sedation. The STA-MCA diameters were 0.8 and 1.2 mm, respectively. After suturing the planned anastomosis with one stitch using an 11-0 monofilament nylon thread, the thread was lifted upward, and the arterial wall was incised. Anastomosis was performed using an 11-0 monofilament nylon thread with 2–4 stitches on each side. The operation was completed without patency problems. Postoperative blood flow improved, and the patient had a good treatment course.
Conclusion: Direct bypass for MMD patients aged
Keywords: Combined bypass surgery, Infant, Moyamoya disease, Superficial temporal artery-to-middle cerebral artery bypass (STA-MCA bypass)
INTRODUCTION
Moyamoya disease (MMD) is a disease of unknown cause characterized by bilateral stenosis of the internal carotid artery endings. It is a chronic, progressive, and obstructive disease in which abnormal blood channels develop as collateral blood channels.[
CASE PRESENTATION
The patient was a 1-year-old girl whose mother and grandmother had a history of MMD. The patient presented with incomplete hemiplegia in the left upper and lower extremities. Magnetic resonance imaging of the head revealed acute cerebral infarction in the white matter of the right cerebral hemisphere [
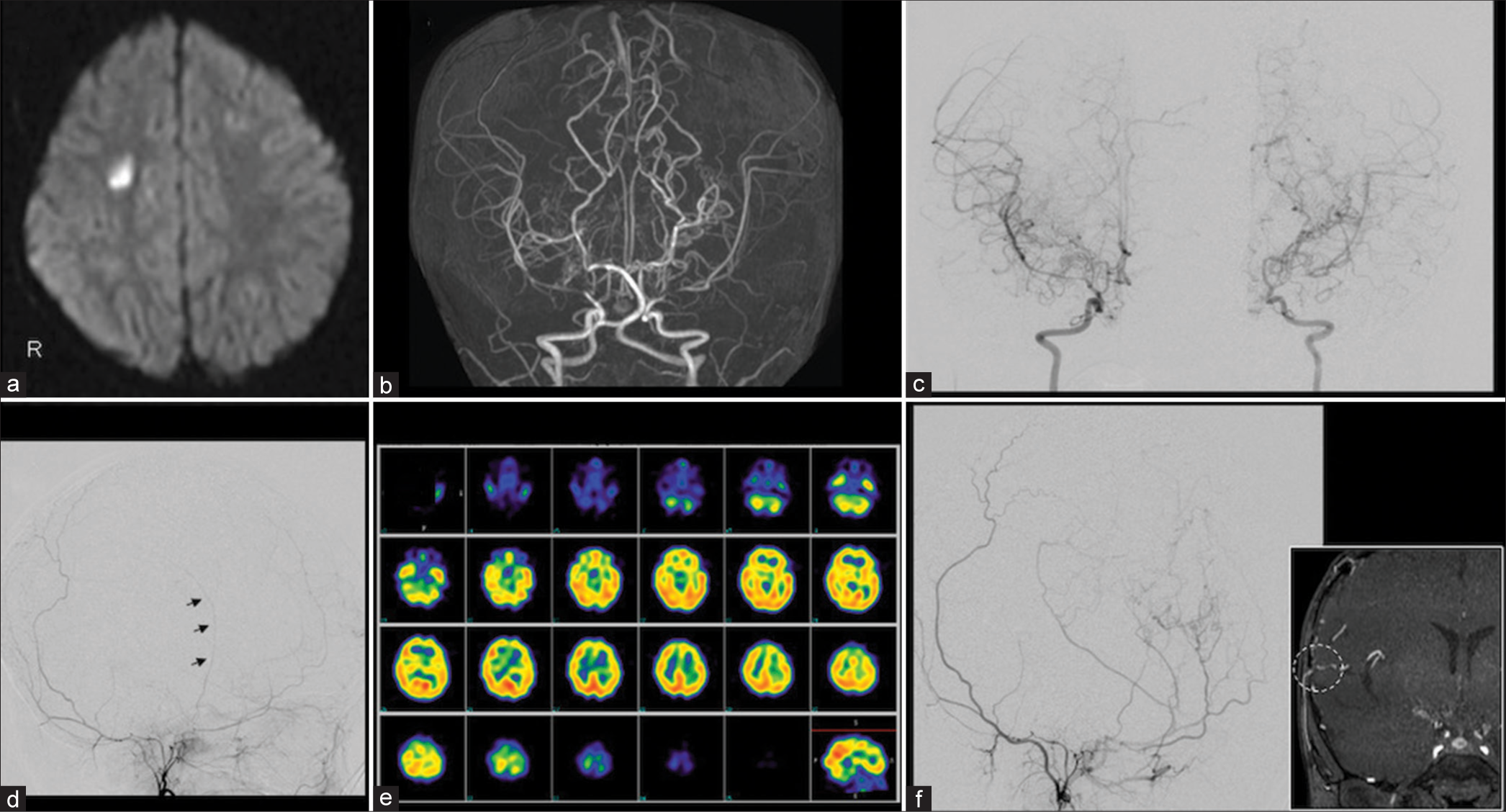
Figure 1:
(a) Magnetic resonance imaging of the head showing acute cerebral infarction in the white matter of the right cerebral hemisphere. (b and c) Magnetic resonance angiography (MRA) of the head and digital subtraction angiography (DSA) show unclear delineation beyond the peripheral portions of the bilateral internal carotid arteries. (d) Left external carotid angiography shows that the superficial temporal artery is very thin (arrows). (e) Single-photon emission computed tomography with N-isopropyl-p-[123I] iodoamphetamine showed reduced blood flow at rest in the bilateral anterior and middle cerebral artery regions. (f) MRA and DSA at three months postoperatively showed good blood flow in the bypass area and collateral blood flow development (arrows, dashed circle).
Three months postoperatively, MRA and DSA showed adequate bilateral blood flow in the bypass area and well-developed collateral blood flow [
Approval was obtained from the University’s Institutional Review Board before initiation of the treatment procedure.
TECHNICAL NOTES
Preoperative preparation
Before treatment, a peripherally inserted central venous catheter (PICC) was inserted to avoid ischemic attacks associated with crying, dehydration, and malnutrition. All examinations and procedures that would be stressful to the patient, such as blood examinations, were performed using the PICC or under sedation [
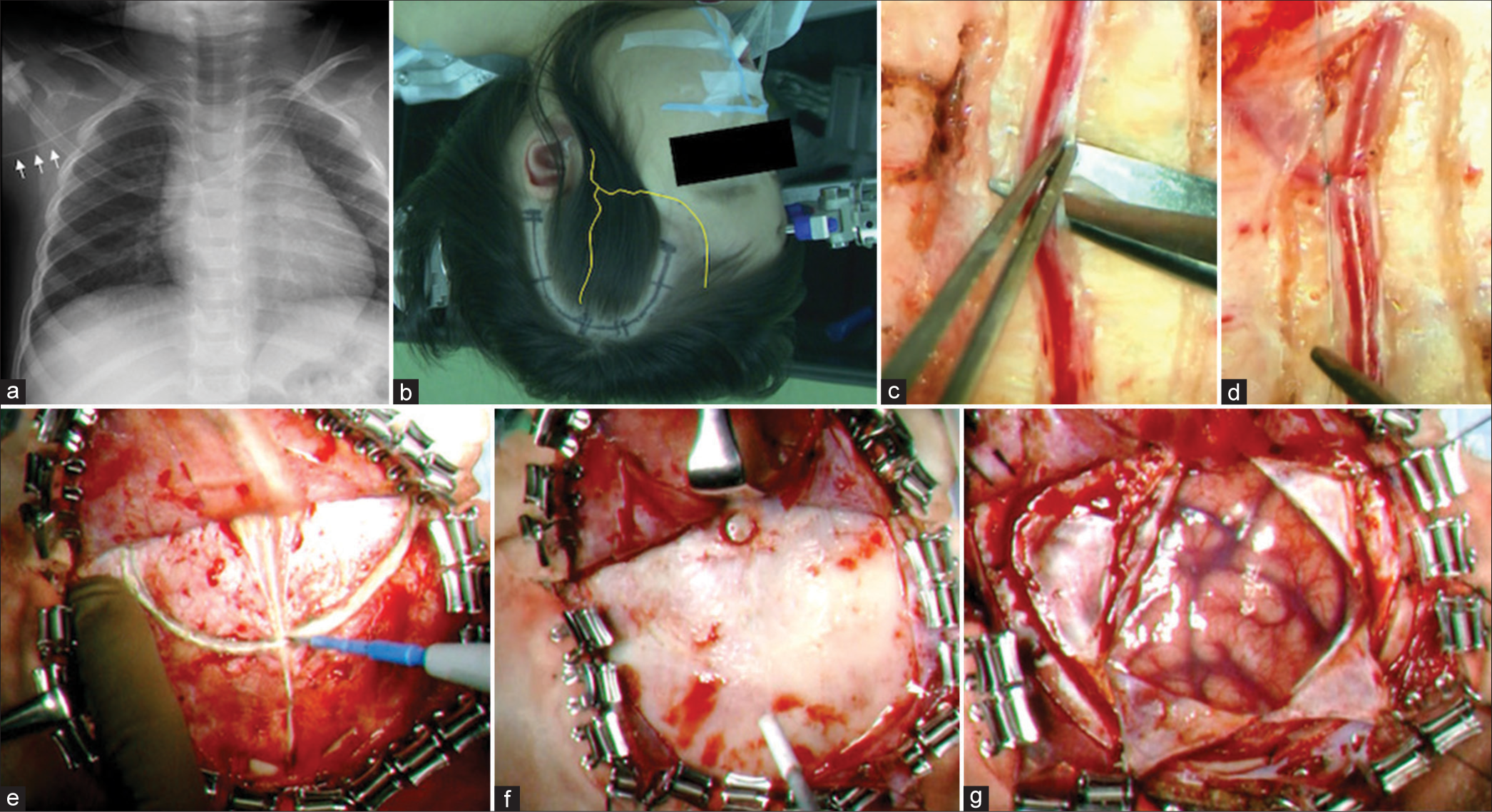
Figure 2:
(a) Before treatment, a peripherally inserted central venous catheter was inserted to avoid ischemic attacks due to dehydration, chirping, and malnutrition (arrows). (b) A semicircular arcuate skin incision was made around the parietal branch of the superficial temporal artery (STA) (the yellow line indicates the STA). (c and d) The STA graft was detached by approximately 4 cm, and the thicker branches were ligated using an 8-0 nylon thread, coagulated and cut. (e) The temporal muscle was incised in the middle along the STA graft run. (f) Craniotomy was performed with only one burr hole for the graft tunnel in children aged <1 year. (g) A cross incision was made in the dura mater to avoid obstructing the passage of the STA graft.
Head fixation
For infants aged <1 year, their skull is thin and should be secured at four points using pediatric pins to reduce the risk of skull penetration [
Video 1
Scalp incision and STA graft harvesting
Using cerebral angiography as a reference, we marked the run of the frontal and parietal branches of the STA on the scalp using palpation and vascular Doppler. To perform a single bypass, a semicircular skin incision was made on a semicircular arc with its base on the auricular side, centered on the parietal branch of the STA, without damaging the main trunk of the frontal branch [
STA grafts were harvested using a microscope. STAs in infants aged <1 year are easily damaged because their diameter is <1 mm and the vessel wall is very thin. However, they are characterized by little flexural tortuosity and vascular branching. Therefore, grafts should be harvested using a combination of low-power coagulation and sharp incision, paying attention to coagulation heat and tear damage during dissection [
Craniotomy and dural incision
The temporal muscle was incised medially along the STA graft run, and a Y-shaped incision was made where the graft passed through the muscle to avoid compression during closure [
The end-tidal CO2 (EtCO2) during surgery should also be noted. Surgery is performed with EtCO2 within normal limits; however, if serious brain swelling is observed when the dura mater is incised, EtCO2 should be lowered.
Recipient preparation
The recipient should be chosen by securing the central or precentral artery of the MCA to distribute bypass blood flow throughout the MCA region as much as possible. After carefully incising the surrounding arachnoid membrane and securing the recipient, a silicone Super Micro Sheet K (Kono Seisakusyo, Tokyo, Japan) was inserted under the recipient to lift the planned anastomosis area to facilitate anastomosis [
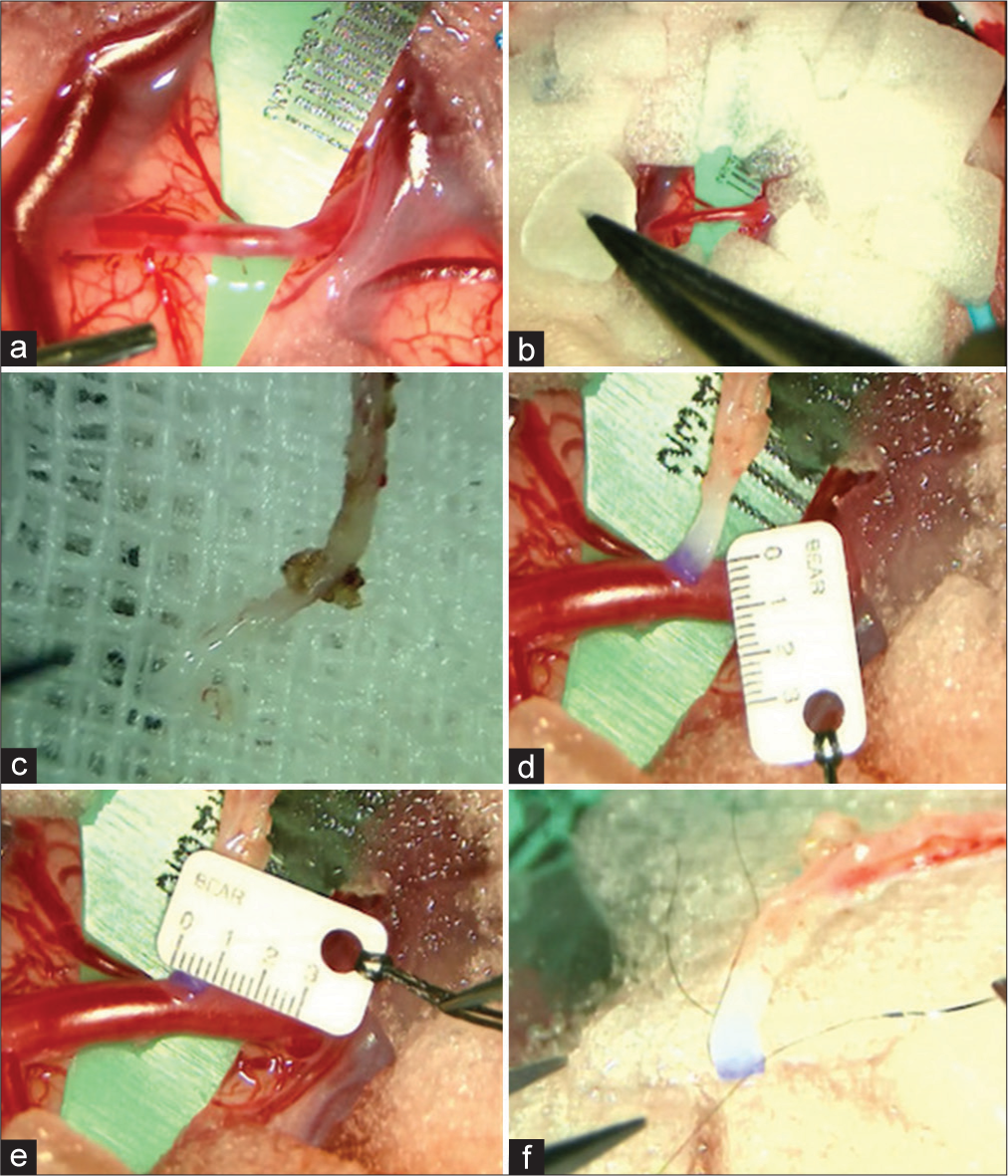
Figure 3:
(a) A silicone super micro sheet K was inserted under the recipient. (b) Gelfoam, cut into small pieces of 10 mm × 10 mm, was placed on the brain surface around the recipient. (c) Approximately 1–2 cm of the tissue attached to the superficial temporal artery (STA) graft end was removed, and the graft tip was trimmed. (d and e) The diameter of the recipient and STA graft was 1.2 mm and 0.8 mm, respectively. (f) Before anastomosis, a stay suture needle was threaded through both ends of the STA graft in advance using an 11-0 monofilament nylon thread.
Patients aged ≤3 Years require caution in anastomosis because the average diameter of the STA graft is 0.8 mm, and the vessel wall is very thin, depending on age. Before anastomosis, a stay suture needle was threaded through both ends of the STA graft in advance using an 11-0 monofilament nylon thread (BEAR Medic Co., Ibaraki, Japan) [
Anastomosis
Because patients with MMD take aspirin, hemostasis of the operative field should be thoroughly checked before anastomosis is performed. After marking the anastomotic site of the recipient and the position where the clip was to be applied with biological pigment, the clip was used to block the blood flow [
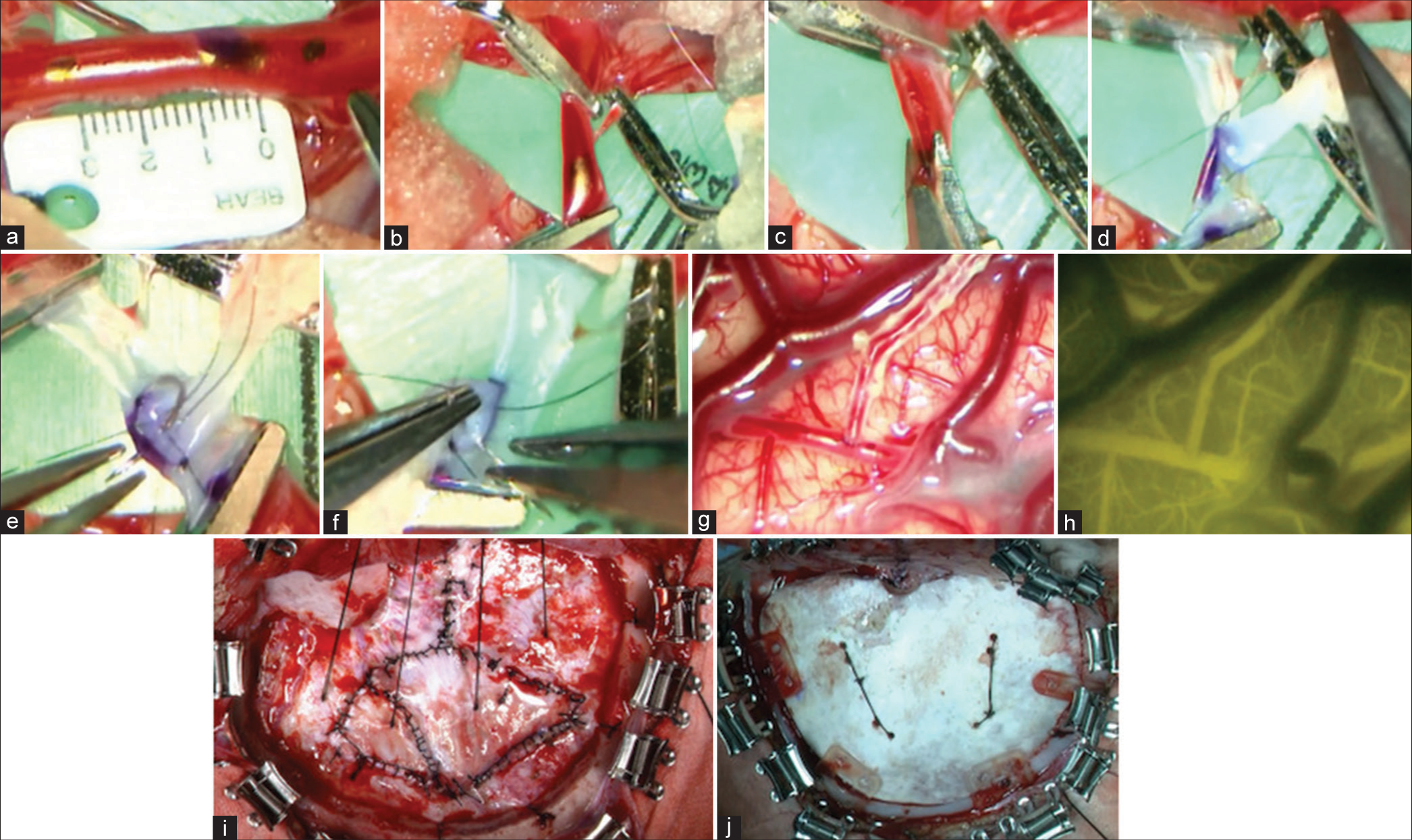
Figure 4:
(a and b) After marking the anastomotic site of the recipient and the position where the clip is to be applied with biological pigment, the clip was used to block blood flow. (c) After suturing the planned anastomosis with one stitch using an 11-0 monofilament nylon thread, the thread was lifted upward, and the arterial wall was incised. (d) A stay suture was first performed at both ends. (e and f) Anastomosis was performed with interrupted sutures. Anastomosis was performed with 2–4 stitches on each side. (g and h) After anastomosis, micro-Doppler and indocyanine green were used to confirm adequate blood flow in the bypass area. (i) The dura mater was folded back to the brain’s surface for encephalo-duro-synangiosis. The dural defect was replaced by harvesting of the temporal fascia. (j) The bone flap was fixed using an absorbable osteosynthesis plate in the infant.
After suturing the planned anastomosis with one stitch using an 11-0 monofilament nylon thread, the thread was lifted upward, and the arterial wall was incised [
From post bypass to closed head
After anastomosis, micro-Doppler and indocyanine green were used to confirm adequate blood flow in the bypass area [
The dura mater is folded back to the brain’s surface for encephalo-duro-synangiosis for indirect bypass. The dural defect was replaced by harvesting of the temporal fascia [
DISCUSSION
We presented a detailed description of STA-MCA bypass surgery in an infant aged <1 year with MMD, focusing on the technical aspects of the procedure and specific surgical procedures. Various revascularization techniques are used for MMD and can be broadly classified into direct and indirect revascularization. Controlled clinical trials have not reported significant differences in outcomes among surgical procedures for pediatric MMD; however, the frequency of ischemic stroke after indirect revascularization has been reported to be 7.7–13.7%.[
When performing direct revascularization for MMD in infants aged <1 year, a PICC should be inserted before treatment, and tests and procedures that may be stressful to the patient should be performed using PICC or under sedation, as appropriate to avoid ischemic attacks associated with crying, dehydration, and malnutrition. Preoperative medical therapy includes aspirin at a dose of body weight × 2 mg/day and continued oral administration during and after surgery.[
CONCLUSION
Direct bypass for MMD patients aged <1 year is technically challenging; however, the vessels can be connected if the procedure is carefully performed, considering the characteristics of the infant’s vessels.
Ethical approval
The research/study was approved by the Institutional Review Board at the Ethical Committee for Epidemiology of Hiroshima University, number E2022-0016, dated February 16, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank the staff of the Department of Neurosurgery for providing clinical assistance.
References
1. Araki Y, Uda K, Yokoyama K, Kanamori F, Kurimoto M, Shiba Y. Challenging direct bypass surgery for very young children with moyamoya disease: Technical notes. Neurosurg Rev. 2022. 45: 1799-807
2. Araki Y, Yokoyama K, Uda K, Kanamori F, Kurimoto M, Shiba Y. Postoperative stroke and neurological outcomes in the early phase after revascularization surgeries for moyamoya disease: An age-stratified comparative analysis. Neurosurg Rev. 2021. 44: 2785-95
3. Bot GM, Burkhardt JK, Gupta N, Lawton MT. Superficial temporal artery-to-middle cerebral artery bypass in combination with indirect revascularization in moyamoya patients ≤ 3 years of age. J Neurosurg Pediatr. 2018. 23: 198-203
4. Funaki T, Takahashi JC, Takagi Y, Kikuchi T, Yoshida K, Mitsuhara T. Unstable moyamoya disease: Clinical features and impact on perioperative ischemic complications. J Neurosurg. 2015. 122: 400-7
5. Hayashi T, Kimiwada T, Karibe H, Shirane R, Sasaki T, Metoki H. Preoperative risks of cerebral infarction in pediatric moyamoya disease. Stroke. 2021. 52: 2302-10
6. Hayashi T, Shirane R, Fujimura M, Tominaga T. Postoperative neurological deterioration in pediatric moyamoya disease: Watershed shift and hyperperfusion. J Neurosurg Pediatr. 2010. 6: 73-81
7. Ibrahimi DM, Tamargo RJ, Ahn ES. Moyamoya disease in children. Childs Nerv Syst. 2010. 26: 1297-308
8. Ishikawa T, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke. 1997. 28: 1170-3
9. Kazumata K, Ito M, Tokairin K, Ito Y, Houkin K, Nakayama N. The frequency of postoperative stroke in moyamoya disease following combined revascularization: A single-university series and systematic review. J Neurosurg. 2014. 121: 432-40
10. Kim SK, Seol HJ, Cho BK, Hwang YS, Lee DS, Wang KC. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery. 2004. 54: 840-4 discussion 844-6
11. Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. 2010. 66: 1093-101 discussion 1101
12. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseas. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 2012. 52: 245-66
13. Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004. 100: 142-9
14. Smith ER, Scott RM. Spontaneous occlusion of the circle of Willis in children: Pediatric moyamoya summary with proposed evidence-based practice guidelines. A review. J Neurosurg Pediatr. 2012. 9: 353-60
15. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
16. Yamada S, Oki K, Itoh Y, Kuroda S, Houkin K, Tominaga T. Effects of surgery and antiplatelet therapy in ten-year follow-up from the registry study of research committee on moyamoya disease in Japan. J Stroke Cerebrovasc Dis. 2016. 25: 340-9