- Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
Correspondence Address:
Naoki Otani
Department of Neurosurgery, National Defense Medical College, Tokorozawa, Saitama, Japan
DOI:10.4103/sni.sni_202_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Naoki Otani, Kojiro Wada, Terushige Toyooka, Satoru Takeuchi, Arata Tomiyama, Kentaro Mori. Usefulness of dural surface tracing of the cortical vessels with indocyanine green videoangiography just prior to dural opening for various cerebrovascular diseases. 22-Aug-2017;8:201
How to cite this URL: Naoki Otani, Kojiro Wada, Terushige Toyooka, Satoru Takeuchi, Arata Tomiyama, Kentaro Mori. Usefulness of dural surface tracing of the cortical vessels with indocyanine green videoangiography just prior to dural opening for various cerebrovascular diseases. 22-Aug-2017;8:201. Available from: http://surgicalneurologyint.com/surgicalint-articles/usefulness-of-dural-surface-tracing-of-the-cortical-vessels-with-indocyanine-green-videoangiography-just-prior-to-dural-opening-for-various-cerebrovascular-diseases/
Abstract
Background:Indocyanine green (ICG) videoangiography can be used to delineate the locations of the cortical vessels just prior to dural opening, allowing safe and optimal dural opening. The present clinical series demonstrates the adjunct use of ICG videoangiography to optimize dural opening for the treatment of various cerebrovascular diseases.
Methods:A total of 45 patients underwent surgery for superficial temporal artery-middle cerebral artery bypass (40), arteriovenous malformation (2), and dural arteriovenous fistula (3) between January 2012 and December 2016. After the dura had been exposed, ICG (0.25 mg/kg) was administered intravenously from the peripheral vein as a bolus just prior to dural opening. The operating microscope equipped with a fluorescent filter was used to examine the illuminated field of interest, and real-time flow assessment of the underlying cortical vessels and/or dural sinus was performed. The target recipient arteries for anastomosis or vascular malformations were visualized through the dura and marked using a pyoktanin pen on the dura mater.
Results:The optimal dural opening was performed for anastomosis, and safety was ensured by locating the vascular malformations through the dura mater in all cases. The cortical vessel injury was avoided in all cases. No complication was related to this procedure.
Conclusions:Dural surface tracing of the cortical vessels with ICG videoangiography just prior to dural opening is a useful technique, which allows optimal and safe dural opening for treatment of various cerebrovascular diseases.
Keywords: Dural opening, ICG videoangiography, microneurosurgery
INTRODUCTION
Indocyanine green (ICG) videoangiography is usually performed during intradural procedures to ensure the safety and completeness of microsurgical procedures for various cerebrovascular diseases (CVDs), by allowing the evaluation of vessel patency and delineation of vascular anatomy during the surgical procedure.[
PATIENTS AND METHODS
The study was approved by the local institutional review committee of the hospital. Patient consent to the study was acquired using a webpage for retrospective and noninvasive study protocol. This retrospective analysis included 45 consecutive patients, 16 women and 29 men aged from 41 to 74 years (mean 57.6 years), treated for STA-MCA bypass (40), AVM (2), and dural AVF (3) between January 2012 and December 2016 at the National Defense Medical College Hospital. Medical charts, radiological findings, complications, and final results were reviewed.
Intraoperative technique
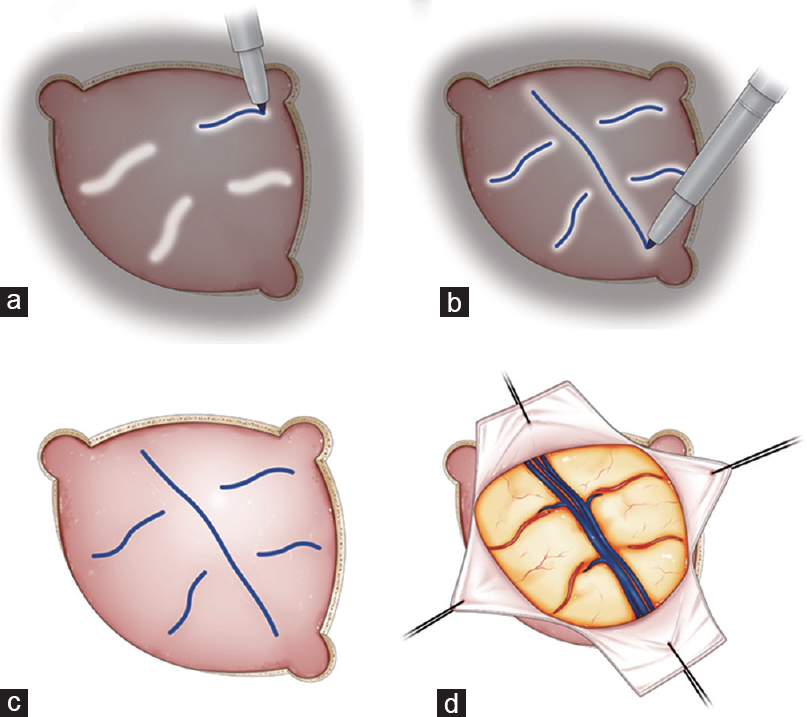
Figure 1
Schematic illustrations showing the surgical procedures of tracing of the cortical vessels on the dural surface with ICG videoangiography. The operating microscope equipped with a fluorescent filter was used to examine the illuminated field of interest, and real-time flow assessment of the underlying cortical vessels and dural sinus was performed (a and b). The dural and cortical vessels were visualized through the dura and marked using a pyoktanin pen on the dura mater (c). Finally, optimal and safe dural incision was performed (d)
RESULTS
The dura was opened and cortical vessel injury was avoided in all cases. Handling of the tools were technically successful during the surgical procedure. No further complication was related to this procedure.
Illustrative cases
Case 1: A patient with symptomatic right M1 stenosis underwent STA-M4 double bypass [
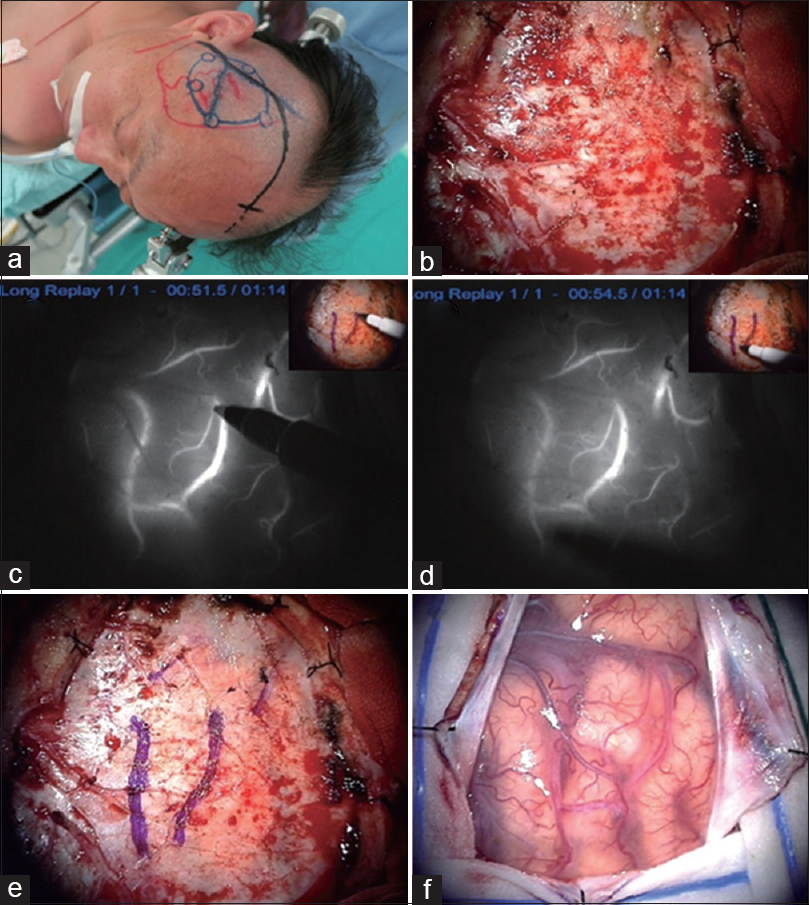
Figure 2
A patient with symptomatic right M1 stenosis underwent STA-M4 double bypass (a). Fronto-temporal craniotomy was planned (b). Just prior to dural opening, ICG (0.25 mg/kg) was administered intravenously. The preoperatively scheduled recipient cortical arteries were visualized through the dura and marked using a pyoktanin pen on the dura mater (c-e). Optimal opening of the dura mater was performed (f)
Case 2: A patient with symptomatic moyamoya disease underwent STA-M4 double bypass [
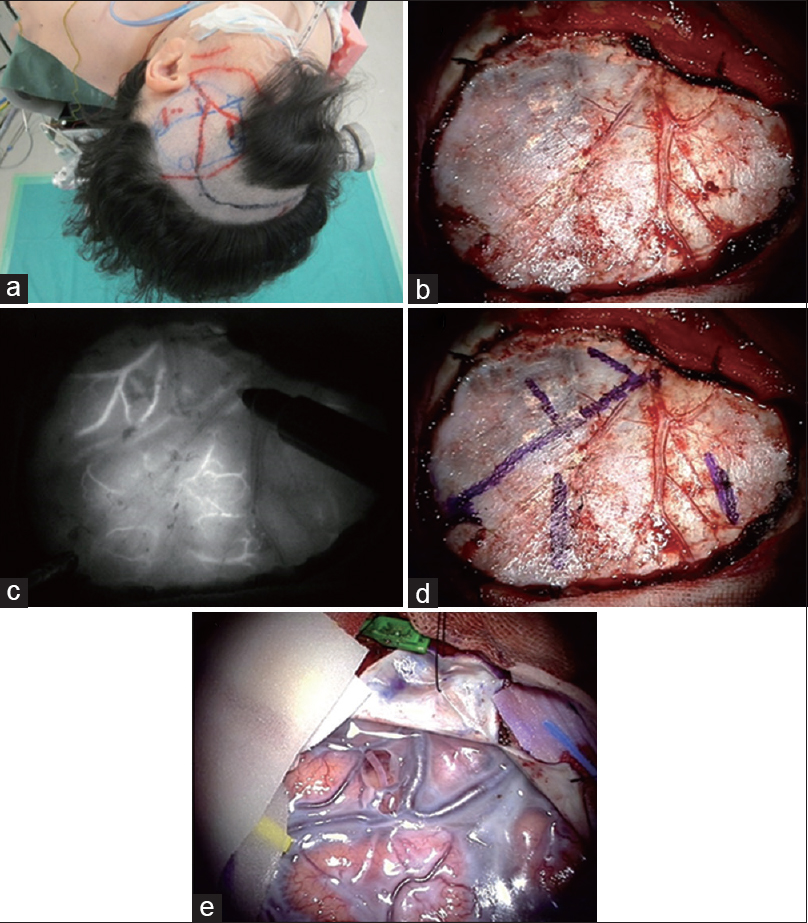
Figure 3
A patient with symptomatic moyamoya disease underwent STA-M4 double bypass (a). Fronto-temporal craniotomy was planned (b). Just prior to dural opening, ICG (0.25 mg/kg) was administered intravenously. The cortical vessels were visualized through the dura and marked using a pyoktanin pen on the dura mater (c and d). The candidates for the recipient cortical arteries were located over the dura mater, and then optimal opening of the dura mater was performed for anastomosis (e)
Case 3: A patient with ruptured AVM underwent removal of the AVM in the acute stage [
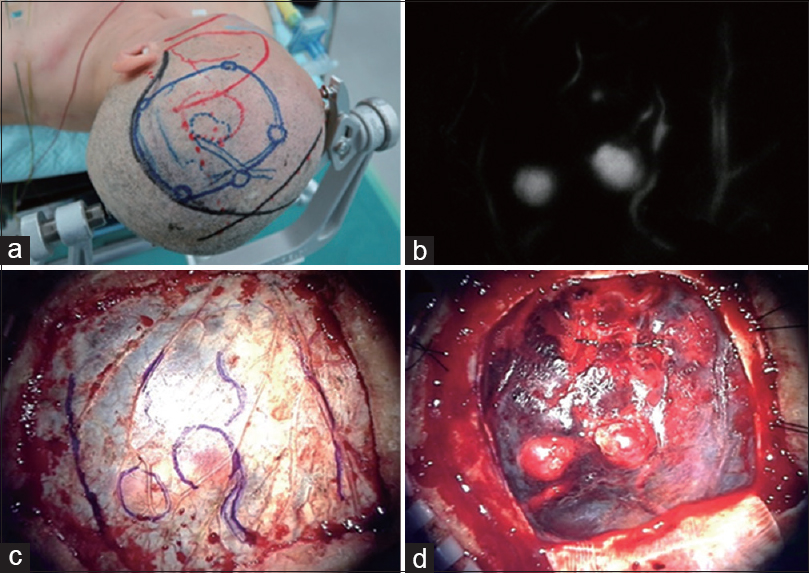
Figure 4
A patient with ruptured AVM underwent removal of the AVM in the acute stage (a). Just prior to dural opening, ICG (0.25 mg/kg) was administered intravenously, and real-time flow assessment of the underlying cortical vessels was performed. The cortically located nidus, feeders, and draining veins were visualized through the dura and marked using a pyoktanin pen on the dura mater (b and c). The dura mater was safely and accurately opened (d)
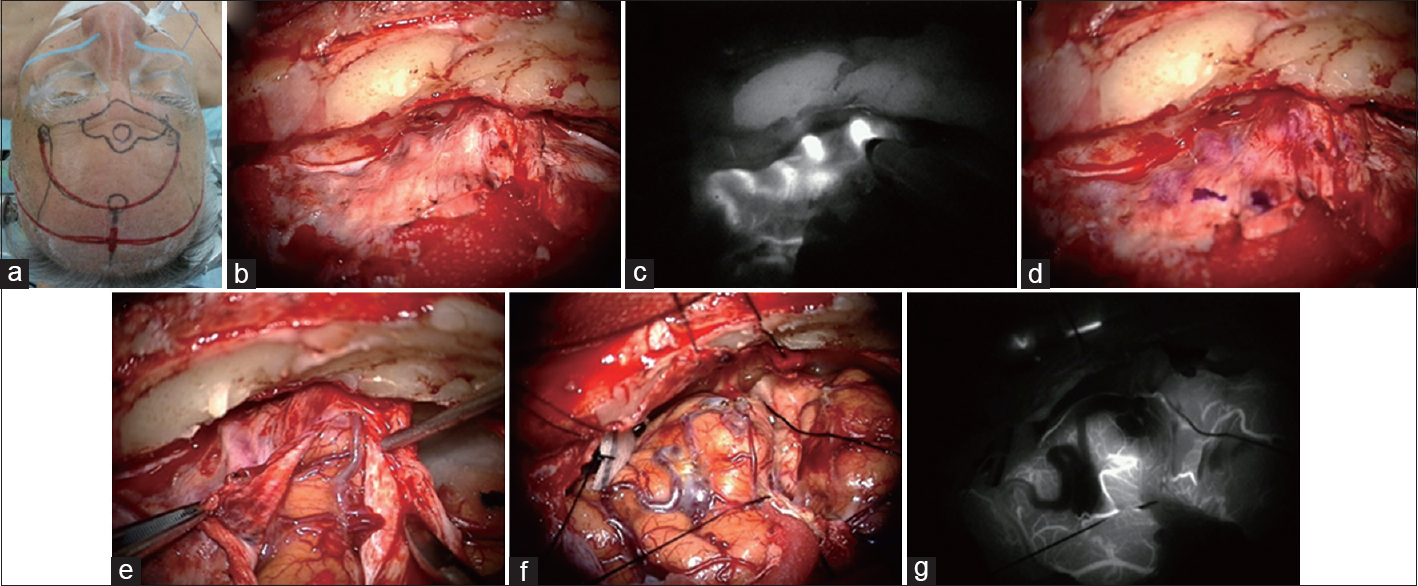
Case 4: A patient with frontal base dural AVF with cortical reflux underwent direct removal [
Figure 5
A patient with dural AVF with cortical reflux underwent direct removal (a). Bicoronal craniotomy was planned (b). Just prior to dural opening, ICG (0.25 mg/kg) was administered intravenously. The varix and cortical draining veins were visualized through the dura and marked using a pyoktanin pen on the dura mater (c and d). The dura mater was safely and accurately opened (e). Varix lesion and the fistula were confirmed to coincide with the findings of ICG videoangiography (f). After resection of the lesion, final ICG videoangiography showed the lesion had disappeared (g)
DISCUSSION
Only two previous studies have evaluated the use of ICG videoangiography prior to dural opening to identify the cortical vessels. ICG videoangiography was used to optimize the dural opening for avoiding cortical vessel injury prior to treatment of parasagittal lesions,[
The present study describes our clinical experiences of dural surface tracing to delineate the locations of the cortical vessels with ICG videoangiography just prior to dural opening for the treatment of various CVDs. For STA-MCA anastomosis, the preoperatively scheduled candidates for the recipient cortical arteries could be visualized through the dura mater, and the extent of dural opening could be optimized to achieve completeness of anastomosis. For removal of AVM or dural AVF, the cortically located nidus, feeders, and varix with draining veins could also be visualized through the dura mater. After marking using a pyoktanin pen on the dura mater, safe and optimal opening could be performed avoiding unexpected cortical vessels injuries.
CONCLUSION
Dural surface tracing of the cortical vessels with ICG videoangiography just prior to dural opening was remarkably useful for performing safe and optimal dural opening for various CVDs.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Dashti R, Laakso A, Niemelä M, Porras M, Hernesniemi J. Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: The Helsinki experience. Surg Neurol. 2009. 71: 543-50
2. Nussbaum ES, Defillo A, Nussbaum L. The use of indocyanine green videoangiography to optimize the dural opening for intracranial parasagittal lesions. Neurosurgery. 2012. 70: 61-3
3. Roessler K, Krawagna M, Dörfler A, Buchfelder M, Ganslandt O. Essentials in intraoperative indocyanine green videoangiography assessment for intracranial aneurysm surgery: Conclusions from 295 consecutively clipped aneurysms and review of the literature. Neurosurg Focus. 2014. 36: E7-
4. Schaller C, Urbach H, Schramm J, Meyer B. Role of venous drainage in cerebral arteriovenous malformation surgery, as related to the development of postoperative hyperperfusion injury. Neurosurgery. 2012. 51: 921-7
5. Torné R, Rodríguez-Hernández A, Lawton MT. Intraoperative arteriovenous malformation rupture: Causes, management techniques, outcomes, and the effect of neurosurgeon experience. Neurosurg Focus. 2014. 37: E12-
6. Woitzik J, Horn P, Vajkoczy P, Schmiedek P. Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg. 2005. 102: 692-8
7. Yokoyama K, Kawanishi M, Yamada M, Tanaka H, Ito Y, Kuroiwa T. Indocyanine green videoangiography for vessel identification and preservation prior to dural opening for microvascular decompression. Turk Neurosurg. 2015. 25: 190-2