- Department of Neurosurgery, Fukuoka Neurosurgical Hospital, Minami-Ku, Fukuoka, Japan.
Correspondence Address:
Shigetoshi Yano, Department of Neurosurgery, Fukuoka Neurosurgical Hospital, 5-3-15 Osa, Minamiku, Fukuoka, Japan.
DOI:10.25259/SNI_965_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shigetoshi Yano, Fumihiro Hiraoka, Hiroya Morita, Hiroto Kawano, Takuto Kuwajima, Shin-ichiro Yoshida, Yoshiaki Hama, Noriaki Tashiro, Shuko Hamaguchi, Hiroshi Aikawa, Yoshinori Go, Kiyoshi Kazekawa. Usefulness of endoscope-assisted surgery under exoscopic view in skull base surgery: A technical note. 11-Feb-2022;13:30
How to cite this URL: Shigetoshi Yano, Fumihiro Hiraoka, Hiroya Morita, Hiroto Kawano, Takuto Kuwajima, Shin-ichiro Yoshida, Yoshiaki Hama, Noriaki Tashiro, Shuko Hamaguchi, Hiroshi Aikawa, Yoshinori Go, Kiyoshi Kazekawa. Usefulness of endoscope-assisted surgery under exoscopic view in skull base surgery: A technical note. 11-Feb-2022;13:30. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11392
Abstract
Background: The use of the exoscope has been increasing in the field of neurosurgery, as it can set the visual axis freely, enabling the surgeon to operate in a comfortable posture. Although endoscope-assisted surgery for compensation of insufficient surgical field is useful under the microscope, we report that using an endoscope in exoscopic surgery is safer and more useful.
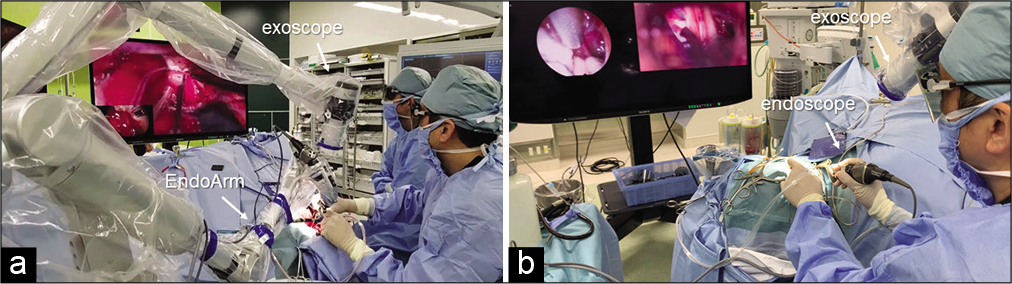
Methods: The exoscope used was ORBEYE. All surgical procedures were performed exoscopically from the beginning of the surgery. When endoscopic observation was required during the operation, the endoscope was inserted under observation by an exoscope. The exoscopic screen was 4K-3D and endoscopic screen was 4K-2D, the operation was performed while observing both screens at the same time. The endoscope was held manually or by a mechanical holder.
Results: Twenty-two cases, including 14 requiring microvascular decompression (MVD) and eight requiring tumor removal, were performed by endoscopic-assisted exoscopic surgery. The endoscope could be inserted safely because its relationship with the surrounding structure could be observed under the exoscope, and the operator could observe both screens without moving the head. Fourteen of 22 patients required additional endoscopic treatment. Satisfactory two-handed operation was performed in 13 cases. Symptomatology disappeared in all cases of MVD, and sufficient tumor resection was achieved.
Conclusion: Exoscopic surgery provides excellent surgical view that is not inferior to conventional microsurgery. As a large space can be secured between the scope and the surgical field, it is safer and easier to manipulate the endoscope under the exoscope.
Keywords: Craniotomy, Endoscopy, Exoscope, Neurosurgery, ORBEYE, Skull base
INTRODUCTION
Endoscope-assisted surgery for observation of blind spots has been introduced in various neurosurgical approaches, and its usefulness has been reported.[
The exoscope currently available in neurosurgical field is a so-called “head-up surgery” system that the surgeon operates while looking at the monitor; this approach has changed the concept of conventional microsurgery. Exoscopes currently used in clinical practice, such as VITOM® 3D (KARL STORZ GmbH & Co., Tuttlingen, Germany), KINEVO 900® (Carl Zeiss AG, Oberkochen, Germany), and ORBEYE® (Sony Olympus Medical Solutions Inc., Tokyo, Japan), all provide high-quality and high-definition (4K-HD) 3-dimensional (3D) images and allow for a wide surgical field due to the long focal length.[
In our hospital, ORBEYE was introduced in November 2018 and used in 216 craniotomies by December 2020. Exoscopic surgery provided a brighter and wider field of view than the microscope, as previously reported,[
MATERIALS AND METHODS
Among the 216 cases of craniotomy performed with an exoscope in our hospital from November 2018 to December 2020, 22 cases of surgeries involving endoscope insertion were included in this study. The endoscope was always placed in the operating room during craniotomy and was used in cases where the surgeon found a blind spot during surgery and required further confirmation. The surgeries included 13 cases of trigeminal neuralgia, one case of hemifacial spasm, five vestibular schwannoma, one trigeminal schwannoma, one petrous edge meningioma, and one tentorial edge meningioma.
The exoscope used in this study was an ORBEYE (Olympus Co., Ltd., Tokyo, Japan). ORBEYE provides 4K-3D images with a small camera head, featuring a focus working distance of 220–550 mm and a maximum magnification of ×12. Head-up surgery was performed on a 55" (140 cm) monitor. In general, a craniotomy was performed under an exoscope from a skin incision. An endoscope was introduced when a blind spot appeared during the operation or when necessary to confirm the operation adequacy.
The endoscope used in this study was an Olympus 4K, 2.7 mm diameter, 30° angled rigid endoscope (Olympus, Tokyo, Japan), which was attached to an EndoArm (Olympus, Tokyo, Japan) or held by hand. The endoscopic images could be observed independently on a 32" (81 cm) monitor, and simultaneously, images from the exoscope and the endoscope were both projected on a 55" (140 cm) monitor for observation. The results of endoscopic surgery during craniotomy with an exoscope were examined.
RESULTS
The insertion of the endoscope during exoscopic surgery was performed without difficulty in all cases. As the endoscope can be inserted while looking at the exoscopic screen, it did not damage any surrounding tissue, a possible complication of typical instrument insertion. The movement of the endoscope during the operation was safely performed by observing the two screens simultaneously.
[
Among the eight cases of tumor resection, two cases were confirmed to have no residual mass in the blind spot such as Meckel’s cave (Case 15) or internal auditory meatus (Case 18) under the endoscope, and additional resection was performed in six cases under the endoscopic observation. In a case of tentorial meningioma, the tumor had spread to the posterior area of the cerebellar tentorium, and the tent was incised and removed tumor under the endoscopic observation. In a case of petroclival meningioma, the tumor that remained in the shadow of the suprameatal tuberculum was removed. In two of three vestibular schwannomas, residual tumors were found in the inferior part of the internal auditory meatus, and additional resection was performed. Two cases of epidermoid cysts had spread to the ventral side of the auditory nerve and were difficult to observe using the exoscope alone; therefore, additional removal was performed under endoscopic view.
Total removal was achieved in seven of eight tumor cases, in the case, the recurrent epidermoid had adhered to the inner part of the brainstem and was not removed.
The EndoArm was used to hold the endoscope in 18 cases. Manual holding was performed in the last four cases (cases 11, 12, 13, and 20). In the 13 cases requiring additional treatment under the endoscope held by the EndoArm, satisfactory two-handed operation was possible in 12 cases, but only one-handed operation was possible in one case (case 8). Of the four recent cases of manual holding, two cases (cases 11 and 20) were held by the operator and two cases (cases 12 and 13) were held by the assistant. In three cases (cases 11, 12, and 13), observation alone was sufficient, and in one case (case 20), the tumor could be removed by suction, which was performed by the surgeon using one hand.
Postoperative cerebrospinal fluid rhinorrhea was observed in one patient (case 2) as a surgical complication due to incomplete blockage of the opened air cells, and surgical repair was performed. No complications associated with endoscopic insertion were observed.
Illustrative cases
[
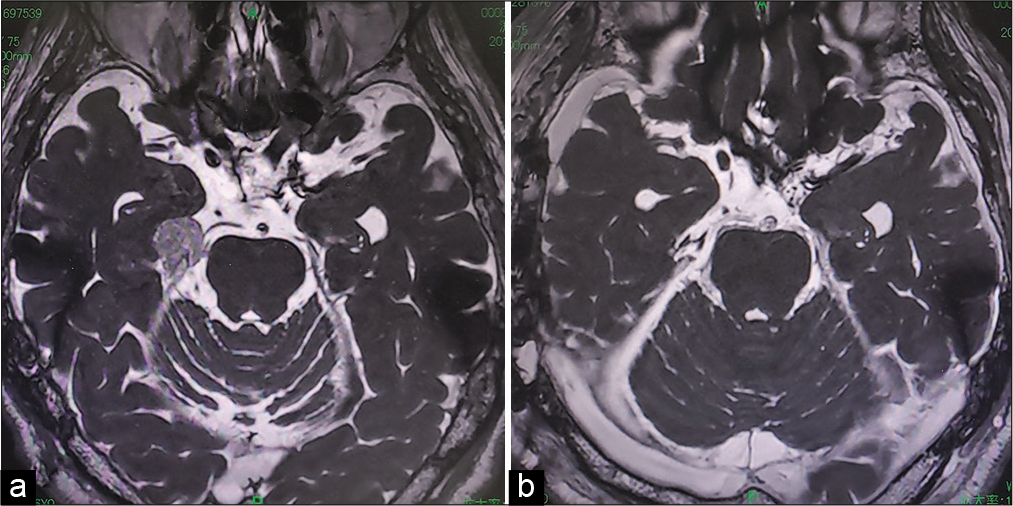
Figure 1:
Pre- and post-operative MRI of illustrative case of a 63-year-old patient with tentorium meningioma. (a) Preoperative MRI thin slice T2-weighted image: a tumor (arrow) is found at the edge of the cerebellar tentorium. (b) Postoperative MRI thin slice T2-weighted image: tumor has been completely resected.
Video 1
[
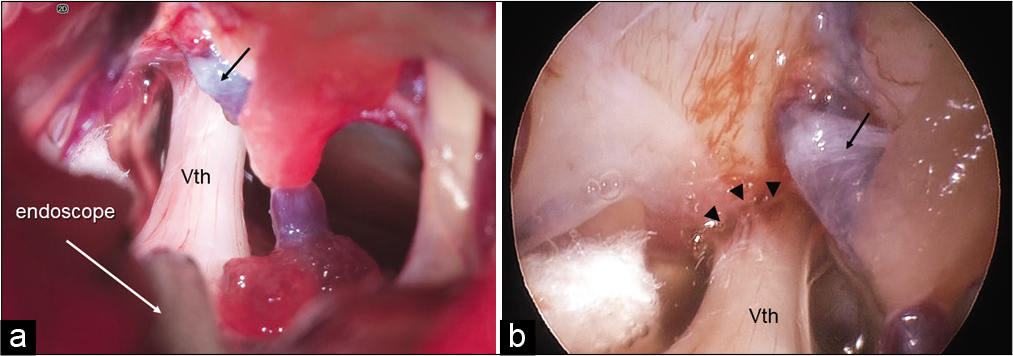
Figure 2:
Intraoperative images of a 73-year-old patient with the right trigeminal neuralgia. (a) Exoscopic image when the endoscope was inserted in the surgical field. As the Meckel’s cave was not able be seen directly, it was difficult to determine whether there was enough space between the Meckel’s cave and vein (arrow). (b) Endoscopic image at the same time as a. The Meckel’s cave (arrow heads) was observed directly and it was confirmed that the adhesions of the arachnoid and the vein (arrow) were released around the Meckel cave of the trigeminal nerve (Vth).
Video 2
DISCUSSION
In recent years, the usefulness of exoscopic surgery in the field of neurosurgery has been reported.[
ORBEYE is a product specifically developed for exoscope and its imaging quality is no less than conventional microscope, and its small size allows effortless handling.[
Endoscopic-assisted craniotomy has been reported in many cases, such as clipping,[
In this respect, exoscopic surgery has a high degree of freedom because a wide operating space can be visualized near the surgical field. Since exoscopic surgery is designed as a head-up surgery, the surgeon can see two screens without moving their head from the scope if the endoscopic screens are arranged in parallel. Therefore, safer endoscopic insertion during operation is possible.
There are various tools for holding an endoscope. EndoArm® (Olympus, Tokyo, Japan), which we used in our study, is an endoscope holder dedicated to neurosurgery.[
In our experience, the ease of two-handed operations while using an endoscope depends on the space between the endoscope and the target structure for the two-handed instrument. It is relatively easy in cases where a large space can be obtained after removal, such as tumor removal, but there are restrictions in narrow range operations, as in the case experienced with MVD, although the most one hand operation was sufficient. In fact, when the EndoArm was held (Case 8), two-handed operation was not possible. The space around the trigeminal nerve was considered to be narrow and the craniotomy opening was small; hence, when the endoscope was inserted from the left, the space for the left hand could not be secured.
In the last four cases (cases 11, 12, and 13), the endoscope was held by hand. When the surgeon becomes accustomed to holding the endoscope, this procedure is effective enough for observation only, and it does not take up much space like EndoArm, and it takes less time to prepare. In one of these cases (case 20), the tumor remaining in the internal auditory meatus could be removed by suction; hence, the operator held the endoscope by the one hand and operated it using the other hand. However, in the case of complicated operations such as dissection and incision, two-handed operation is indispensable, and in that case, we have to fix the endoscope to the EndoArm.
Because it is better to hold the endoscope by hand for fine adjustment during operation, handling of endoscope by a skilled “scopist” like an assistant doing four hands approach during endonasal endoscopic surgery[
Since skull base surgery requires deep manipulation, it is expected that blind spots will be created more frequently than surgery at other sites. For such situations, it is important that the endoscope is always available in the operating room. In exoscopic surgery, the endoscope can be inserted safely without stress, so the combined use of both is considered to be extremely useful.
CONCLUSION
Exoscopic surgery provides excellent surgical view that is not inferior to conventional microsurgery. As a large space can be secured between the scope and the surgical field, it is safer and easier to manipulate the endoscope under the exoscope.
Ethical approval
This study has been approved by the appropriate ethics committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
References
1. Abolfotoh M, Bi WL, Hong CK, Almefty KK, Boskovitz A, Dunn IF. The combined microscopic-endoscopic technique for radical resection of cerebellopontine angle tumors. J Neurosurg. 2015. 123: 1301-11
2. Almeida JP, de Albuquerque LA, Dal Fabbro M, Sampaio M, Medina R, Chacon M. Endoscopic skull base surgery: Evaluation of current clinical outcomes. J Neurosurg Sci. 2019. 63: 88-95
3. Broggi M, Acerbi F, Ferroli P, Tringali G, Schiariti M, Broggi G. Microvascular decompression for neurovascular conflicts in the cerebello-pontine angle: Which role for endoscopy?. Acta Neurochirurg. 2013. 155: 1709-16
4. Cheng WY, Chao SC, Shen CC. Endoscopic microvascular decompression of the hemifacial spasm. Surg Neurol. 2008. 70: 40-6
5. El Refaee E, Langner S, Marx S, Rosenstengel C, Baldauf J, Schroeder HW. Endoscope-assisted microvascular decompression for the management of hemifacial spasm caused by vertebrobasilar dolichoectasia. World Neurosurg. 2019. 121: e566-75
6. Eskandari R, Amini A, Yonemura KS, Couldwell WT. The use of the Olympus EndoArm for spinal and skull-based transsphenoidal neurosurgery. Minim Invasive Neurosurg. 2008. 51: 370-2
7. Fischer G, Oertel J, Perneczky A. Endoscopy in aneurysm surgery. Neurosurgery. 2012. 70: 184-90
8. Gallieni M, Del Maestro M, Luzzi S, Trovarelli D, Ricci A, Galzio R. Endoscope-assisted microneurosurgery for intracranial aneurysms: Operative technique, reliability, and feasibility based on 14 years of personal experience. Acta Neurochirurg Suppl. 2018. 129: 19-24
9. Langer DJ, White TG, Schulder M, Boockvar JA, Labib M, Lawton MT. Advances in intraoperative optics: A brief review of current exoscope platforms. Oper Neurosurg (Hagerstown). 2020. 19: 84-93
10. Liu JK, Dodson VN. Endoscopic-assisted microvascular decompression of ectatic vertebral artery for hemifacial spasm: Operative video and technical nuances. J Neurol Surg B Skull Base. 2019. 80: S312-S3
11. Luzzi S, Gallieni M, Del Maestro M, Trovarelli D, Ricci A, Galzio R. Giant and very large intracranial aneurysms: Surgical strategies and special issues. Acta Neurochir Suppl. 2018. 129: 25-31
12. Magnan J. Endoscope-assisted decompression of facial nerve for treatment of hemifacial spasm. Neurochirurgie. 2018. 64: 144-52
13. Murai Y, Sato S, Yui K, Morimoto D, Ozeki T, Yamaguchi M. Preliminary clinical microneurosurgical experience with the 4K3-dimensional microvideoscope (ORBEYE) system for microneurological surgery: Observation study. Oper Neurosurg (Hagerstown). 2019. 16: 707-16
14. Oertel JM, Burkhardt BW. Vitom-3D for exoscopic neurosurgery: Initial experience in cranial and spinal procedures. World Neurosurg. 2017. 105: 153-62
15. Peris-Celda M, Da Roz L, Monroy-Sosa A, Morishita T, Rhoton AL. Surgical anatomy of endoscope-assisted approaches to common aneurysm sites. Neurosurgery. 2014. 10: 121-44
16. Rak R, Sekhar LN, Stimac D, Hechl P. Endoscope-assisted microsurgery for microvascular compression syndromes. Neurosurgery. 2004. 54: 876-81
17. Rossini Z, Cardia A, Milani D, Lasio GB, Fornari M, D’Angelo V. VITOM 3D: Preliminary experience in cranial surgery. World Neurosurg. 2017. 107: 663-8
18. Sack J, Steinberg JA, Rennert RC, Hatefi D, Pannell JS, Levy M. Initial experience using a high-definition 3-dimensional exoscope system for microneurosurgery. Oper Neurosurg (Hagerstown). 2018. 14: 395-401
19. Todeschini AB, Montaser AS, Shahein M, Revuelta JM, Otto BA, Carrau RL. Endoscopic endonasal approach to a suprasellar craniopharyngioma. J Neurol Surg B Skull Base. 2018. 79: S237-8
20. Tuchman A, Platt A, Winer J, Pham M, Giannotta S, Zada G. Endoscopic-assisted resection of intracranial epidermoid tumors. World Neurosurg. 2014. 82: 450-4
21. Yousaf J, Afshari FT, Ahmed SK, Chavda SV, Sanghera P, Paluzzi A. Endoscopic endonasal surgery for clival chordomas a single institution experience and short term outcomes. Br J Neurosurg. 2019. 33: 388-93