- Department of Neurology, King Abdulaziz Medical City, Riyadh, Saudi Arabia,
- Department of Neurosurgery, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia,
- Department of Neurosurgery, King Abdulaziz Medical City, Riyadh, Saudi Arabia,
- Department of Anesthesiology, Lozano Blesa University Clinical Hospital, Zaragoza, Spain.
- Department of Neurology, Lozano Blesa University Clinical Hospital, Zaragoza, Spain.
Correspondence Address:
Turki Elarjani
Department of Neurosurgery, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_431_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Vizmary Montes1, Turki Elarjani2, Sami Khairy1, David Pinilla3, Helena Benito4, Estella Llado5. Valuableness of introduction of laryngeal abductor reflex intraoperative neuromonitoring technique in lower brainstem lesion. 11-Dec-2020;11:425
How to cite this URL: Vizmary Montes1, Turki Elarjani2, Sami Khairy1, David Pinilla3, Helena Benito4, Estella Llado5. Valuableness of introduction of laryngeal abductor reflex intraoperative neuromonitoring technique in lower brainstem lesion. 11-Dec-2020;11:425. Available from: https://surgicalneurologyint.com/surgicalint-articles/10451/
Abstract
Background: Our aim is to evaluate the use of laryngeal adductor reflex (LAR) for posterior fossa and brainstem surgeries in conjunction with current intraoperative neuromonitoring (IONM) techniques.
Case Description: The patient is a 62-year-old woman who complained of decreased hearing on her left side, dizziness, and left facial palsy. After proper investigation, she was found to have a left vestibular schwannoma. She was scheduled for the left retrosigmoid approach and electrodes embedded on the surface of the endotracheal tube were inserted to monitor for LAR. Preoperative baseline monitoring was recorded. During intraoperative resection of tumor, a significant bilateral amplitude response decrease of the LAR was noted, along with left side decrease in vocal muscle motor evoked potential amplitude responses and bradycardia. Following the LAR event, owed to numerous other IONM changes, surgery was terminated to avoid any complications.
Conclusion: LAR is an integral tool to constantly monitor vagus nerve function that can be used in combination with other IONM modalities during lower brainstem and posterior fossa surgeries. We advocate the IONM use of LAR in brainstem surgeries.
Keywords: Brainstem surgery, Intraoperative neuromonitoring, Laryngeal adductor reflex, Posterior fossa surgery
INTRODUCTION
Intraoperative neuromonitoring (IONM) recording of the laryngeal adductor reflex (LAR) is a newly developed technique mainly used in head-and-neck surgeries. LAR is a brainstem-modulated involuntary airway protective reflex arc to stimuli on the laryngeal mucosa, preventing foreign material from inappropriately entering the upper airway. The superior laryngeal nerve (SLN) functions as the afferent limb and the recurrent laryngeal nerve (RLN) as this reflex’s efferent limb, resulting in vocal fold closure. This technique has been used in thyroid surgery and showed promising results in regard to the RLN and vagus nerve (VN) monitoring.[
CASE REPORT
A 62-year-old woman with a medical history of nondestructive surgical treatment of Meniere’s disease 25 years ago and moderate-to-severe bilateral sensorineural hearing loss, greater for the left ear and using hearing aid on the right ear, who presented to the emergency room due to 3 months history of progressive gait instability, dizziness, and left mouth angle weakness associated with 2-week history of reduced sensitivity in the left facial region. Physical examination revealed left facial paresis (House-Brackmann Grade 2) and left facial hypoesthesia in the territory of the maxillary branch of the trigeminal nerve.
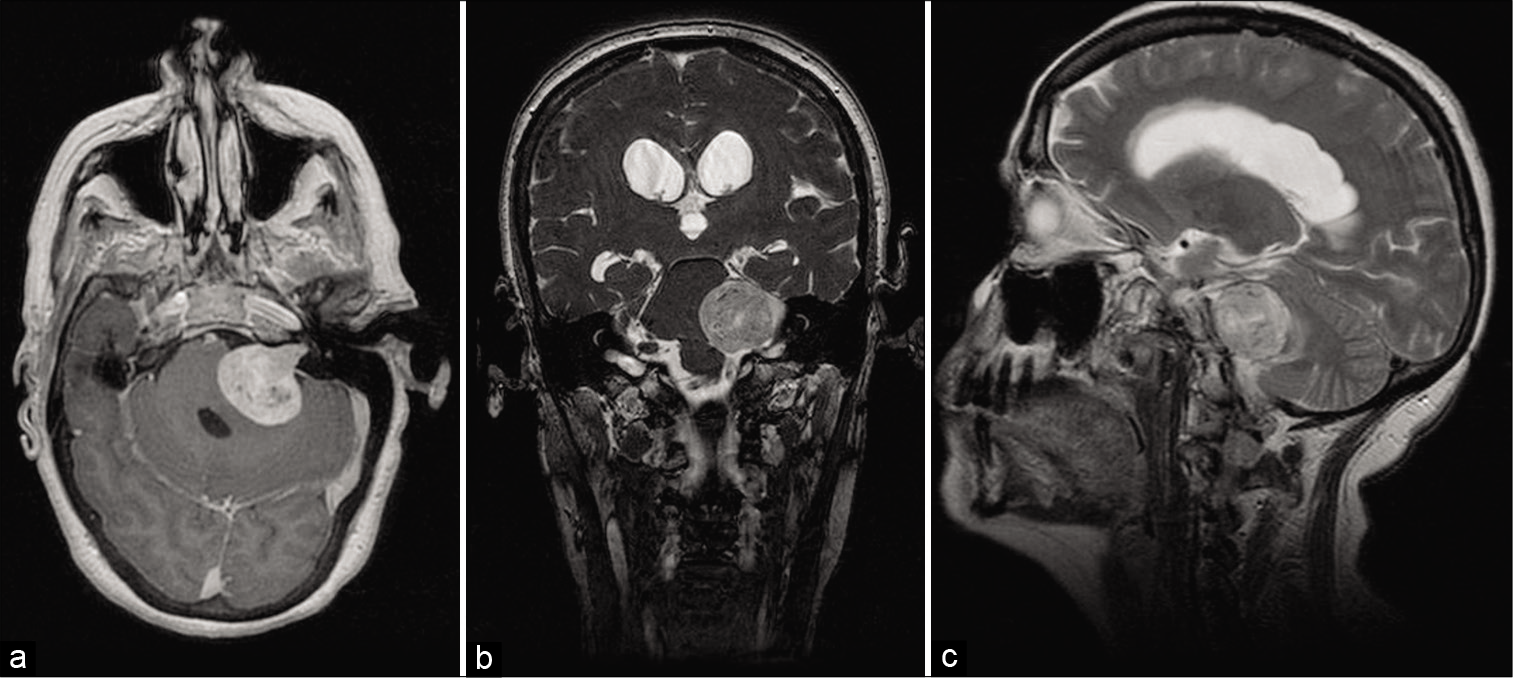
An urgent brain computed tomography (CT) scan with and without contrast was requested, showing evidence of heterogeneously enhancing 3x2 cm space-occupying lesion at the left cerebellopontine angle (CPA). The patient was admitted, and a brain magnetic resonance imaging study was requested, revealing a large 30 (AP) × 25 (T) × 30 (CC) mm size enhancing (T1W) hyperintense (T2W) well-defined mass within the left CPA with intra- and extracanalicular extension. The lesion exerts mass effect on the 4th ventricle with marked dilatation of the third and bilateral lateral ventricles. The lesion was consistent with the left side vestibular schwannoma [
Figure 1:
Preoperative magnetic resonance imaging of the brain axial post contrast-enhanced T1W image (a), coronal T2W image (b), and sagittal T2W image (c) showing a large 30 (AP) × 25 (T) × 30 (CC) mm size hyperintense well-defined mass within the left CPA with intraand extracanalicular extension. The tumor exerts mass effect on the 4th ventricle, with marked dilatation of the third and bilateral lateral ventricles.
Surgical procedure
Anesthesia regimen consisted of total intravenous anesthesia (propofol and remifentanil), and muscle relaxants/ rocuronium were only used during induction. Blood pressure (BP) and heart rate (HR) on arrival to the operating room were 120/75 mmHg and 63 beats/min. After induction, the patient was intubated with an NIM TriVantage electromyography (EMG) endotracheal tube size 7 mm (Medtronic™, Xomed Inc., Jacksonville, Florida) for bilateral monitoring of VN. Intubation and tube positioning were done using video laryngoscope, alongside with the neurophysiologist to ensure optimal electrode depth, alignment, and direct contact with the right and left vocal cords. Midline fixation of the tube using bite blocks followed with the tape-tying method was done to prevent tube rotation and upward or downward displacements of the electrodes during the operation. Posttube intubation and posttube positioning left side cR1 and right side cR1 and cR2 responses were present and stable bilaterally.
After the patient intubation, electrodes were placed bilaterally for upper and lower limb somatosensory evoked potentials (SSEPs) and muscle motor evoked potentials (mMEPs). Electrodes for free-run EMG, brainstem mapping (BSM), and corticobulbar motor evoked potentials (CoMEPs) were placed on the left masseter, orbicularis oculi, orbicularis oris, soft palate, trapezius, and tongue muscles to monitor cranial nerves V, VII, IX, XI, and XII, respectively. Left blink reflex (BR) responses were also recorded by stimulating the left supraorbital nerve and recording from the left orbicularis oculi muscle.
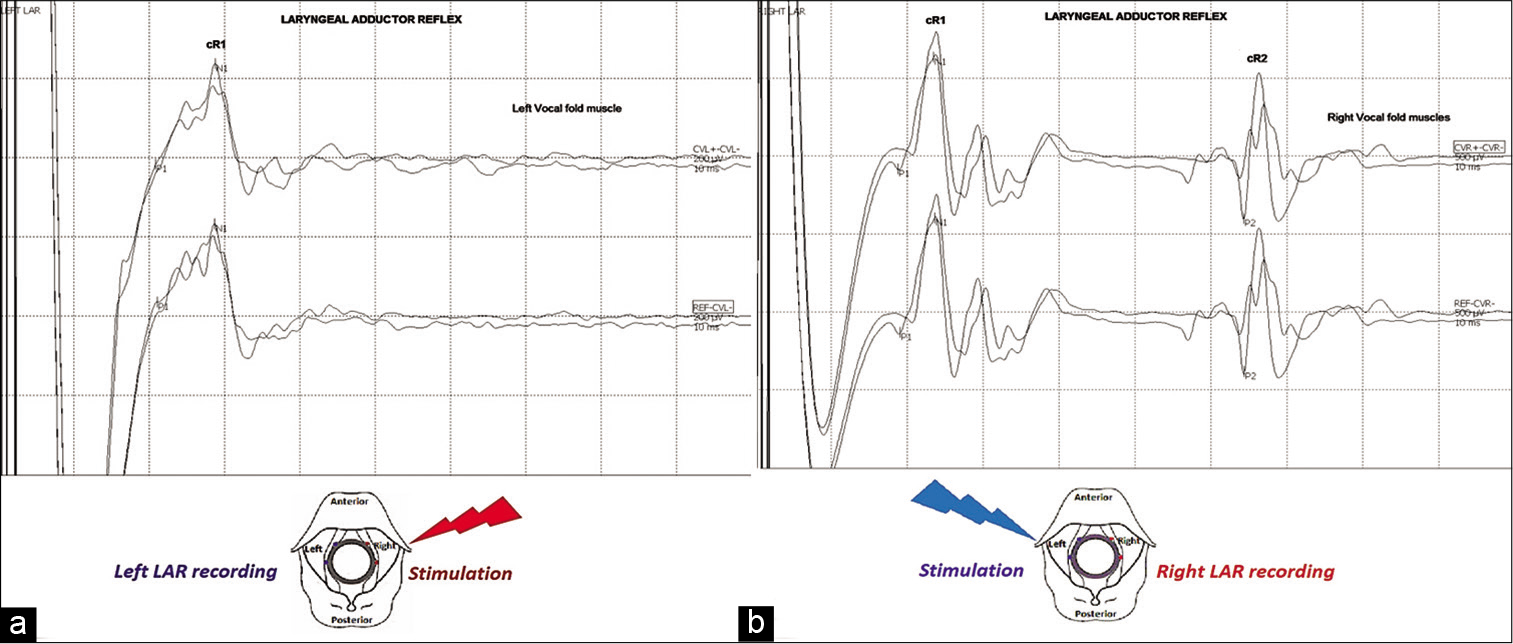
The LAR was evoked by independent stimulation of the right and left side of the laryngeal mucosa (single electrical stimulus, duration 0.5–0.7 ms, intensity 3–5 mA) using embedded EMG tracheal tube electrodes. Contralateral LAR responses (cR1 and cR2) were recorded using endotracheal tube electrodes positioned contralaterally to the stimulating side. The monitoring parameter for IONM interpretation of this modality consisted of considerable attenuation (≥60%) in LAR amplitude responses [
Figure 2:
Baselines for (a) laryngeal adductor reflex (LAR) left side cR1 (ipsilateral side to the lesion) and (b) LAR right side cR1 and cR2 responses, which are shown at top of both figures, respectively. Schematic illustration of LAR methodology is shown at the bottom. Embedded electromyography tracheal tube electrodes, left (blue) and right (red), are positioned directly with the left and right vocal folds. LAR is evoked through electrodes positioned contralaterally to the surgical field, stimulating independently the right and left side of the laryngeal mucosa. LAR responses (cR1 and cR2) are recorded using endotracheal tube electrodes positioned contralaterally to the stimulating side.
Postpositioning SSEPs, mMEPs, and CoMEPs responses were present in all extremities and left side corticobulbar targeted muscles. The left BR responses were stable and with proper morphology. Post patient positioning baselines for LAR left side cR1 and right side cR1 and cR2 responses were good and reliable. Preoperative and intraoperative brainstem auditory evoked potential waves were absent, and the surgeon was informed of baseline responses.
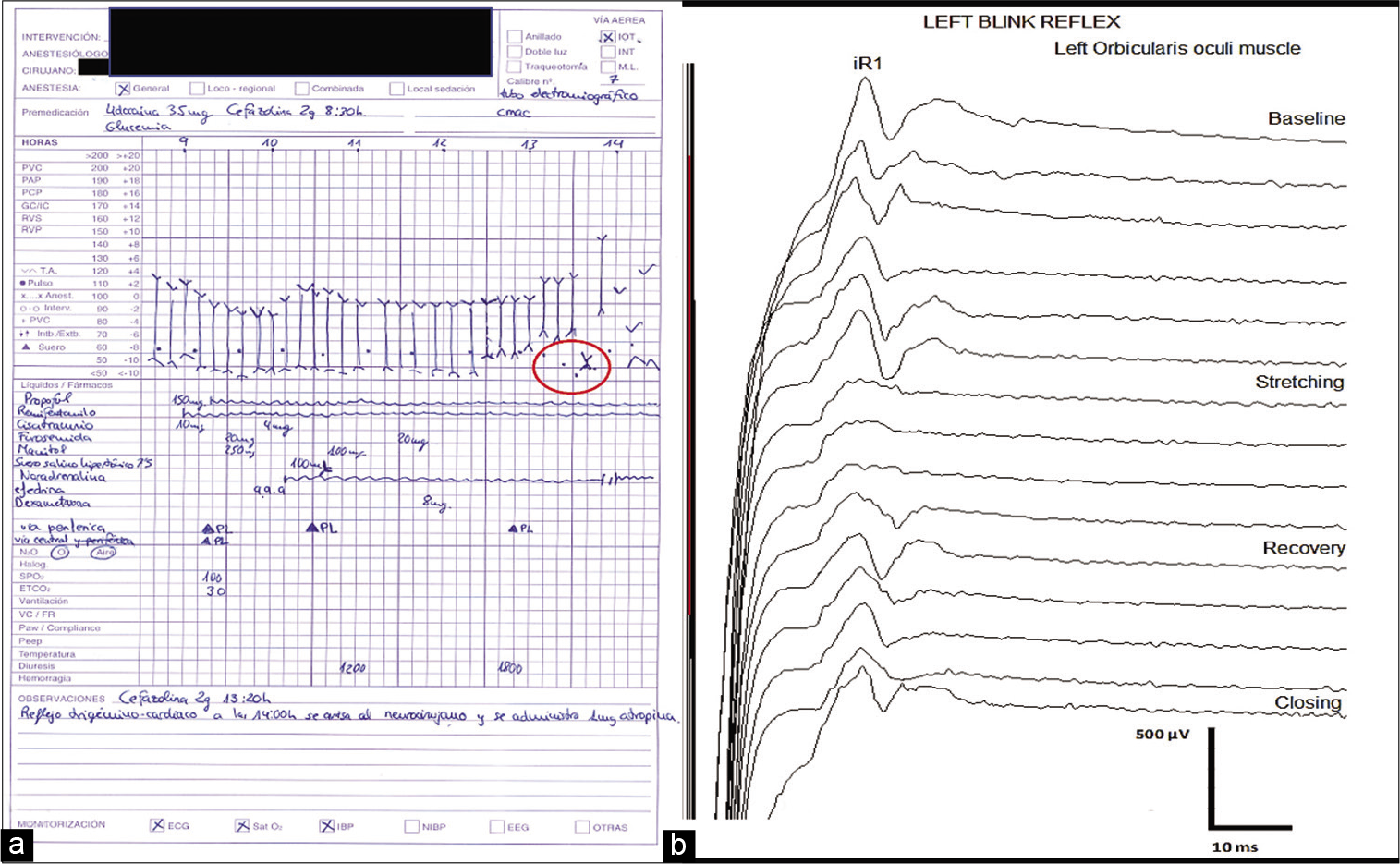
The dura was opened with adequate surgical corridor and exposure; the tumor was visualized along with brainstem and lower cranial nerves; BSM was then performed. During tumor resection, the patient’s HR and BP dropped significantly. IONM changes related to the event consisted of reduced left-sided BR amplitude responses with no other alterations. The surgeon was informed, and surgical stimulation was discontinued. A dose of 1.0 mg atropine sulfate was administered to the patient to counteract the bradycardia, with returning of HR and BR responses to baseline values within 1 min after its administration [
Figure 3:
Sudden drop of heart rate (HR) (●) and blood pressure (BP) (↕) values from baseline (a), which correlates with surgical removal of tumor adjacent to the left trigeminal nerve. The anesthetic chart shows how HR and BP recovered to baseline after surgical stimulation was discontinued. Blink reflex (BR) intraoperative neuromonitoring changes (b) showed decrease of the left BR iR1 from baseline coinciding timely with stretching of the left trigeminal nerve and hemodynamic disturbances. The reflex recovered to baseline after surgical stimulation was discontinued.
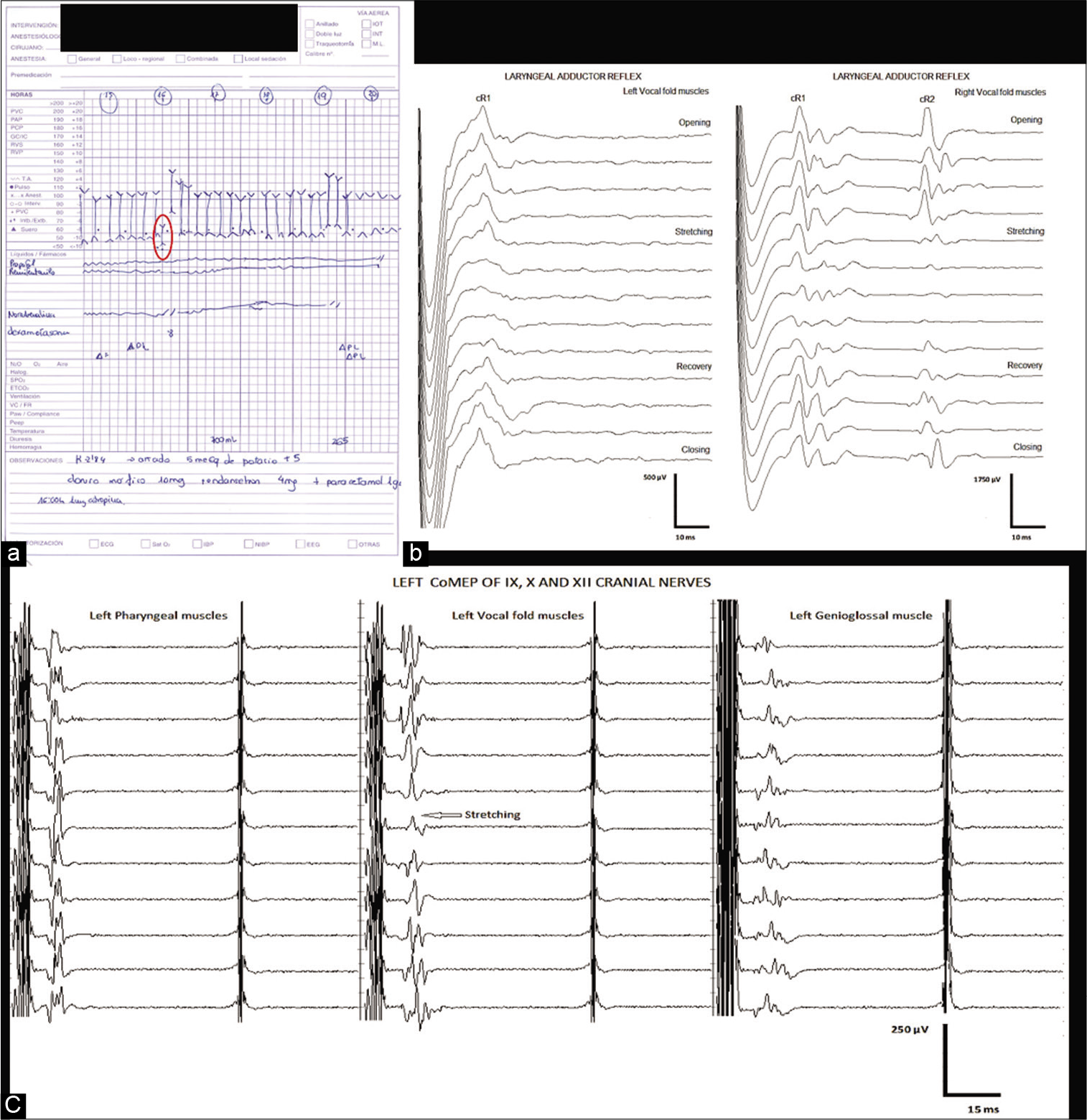
A second event occurred soon after while continuing with tumor resection alongside lower cranial nerves. HR and BP significantly decreased throughout the event, and a significant amplitude response decrease was seen not only on left LAR cR1 but also on right LAR cR1 and cR2 responses, attesting the bilateral nature of this reflex. An amplitude response decrement over the left-sided vocal CoMEP was evident, alongside with neurotonic discharges in the left side X cranial nerve. No other IONM cranial nerve changes seen throughout this event. The surgical team acted immediately, neural stimulation was stopped, and 1.0 mg of atropine was given to the patient immediately, with improvement of hemodynamic parameters, bilateral LAR, and left side CoMEP responses 1 min after administering atropine [
Figure 4:
Sudden drop of heart rate (HR) (●) and blood pressure (BP) (↕) from baseline (a). Consecutive traces of the left and right laryngeal adductor reflex (LAR) (b) showing reversible reduction of both cR1 (left traces) and cR1 and cR2 (right traces) responses from baseline and simultaneous reversible reduction of the left vocal corticobulbar motor evoked potentials (CoMEPs) responses (c). All these changes correlated temporally with surgical maneuvers that stretched the left vagus nerve. All HR, BP, left-sided vocal CoMEP, and bilateral LAR side responses recovered to baseline after surgical stimulation was discontinued.
During dissection of the tumor adjacent to the left facial nerve, frequent and prolonged trains of neurotonic discharges were observed in all branches of the left side VII cranial nerve. Due to several IONM and hemodynamic changes during the procedure and considering that more than 90% of tumor resection was accomplished, tumor resection was discontinued. BSM, mMEPs, and SSEPs remained normal during tumor resection.
The patient was transferred to the ICU for strict neurological monitoring and observation. Forty-eight hours postoperatively, noncontrast brain CT was requested and showed no postoperative complications. The patient was transferred to the neurosurgery ward.
Patient outcome and follow-up
The left sensorineural hearing loss persisted, in addition to horizontal nystagmus. There was worsening of left-sided facial nerve paresis with involvement of the orbicularis oris and complete eyelid closure with maximum effort (House-Brackmann Grade 3), which required covering of the left eye at night and application of eye drops. The patient also presented with mild dysphagia that required adjustment of soft diet; dysphagia resolved gradually within the next 48 h. There were no other neurological deficits of the remaining lower cranial nerves, descending and ascending neural tracts.
Rehabilitation therapy was initiated at 2 weeks after surgery with marked improvement at week 4 postoperatively. At 1-month follow-up, the patient can close her eye with minimal effort, and her mouth showed mild asymmetry (House-Brackmann Grade 2). Conclusive pathological study of the tumor showed Schwannoma-WHO Grade I.
DISCUSSION
LAR is a primitive brainstem reflex that protects the tracheobronchial tree from foreign materials. The reflex represents the bilateral response of the thyroarytenoid muscle from different stimulation types of the laryngeal mucosa. The sensory inputs are carried through the internal branch of the SLN; the signals then travel to the nucleus ambiguous, which controls laryngeal muscle adduction through efferent RLN.[
Mechanism of LAR intraoperative monitoring is through stimulation of glottis/supraglottic receptors contralateral to the operative field and recording of efferent activities on the ipsilateral side of the operative field.[
The LAR R1 responses can be divided into ipsilateral and contralateral responses. The contralateral response is the frequently used recording intraoperatively with a mean latency of 22.4 ms and amplitude of 243.4 mV.[
Continuous nerve monitoring predicts early stretch or pressure injury to nerve fibers intraoperatively and avoids permanent deficits. Sinclair et al. noted a decrement of >60% in LAR opening-closing amplitude or <100 mV amplitude is predictive of nerve irritation and injury if not addressed promptly (P < 0.001).[
Few letters to the editor articles were published in the literature regarding the use of LAR in lower brainstem surgeries. Costa et al. showed beneficial results in three pediatric patients who underwent lower brainstem surgeries with multimodal IONM.[
In our patient, the hemodynamic perturbation occurred while continuing with tumor resection near lower cranial nerves and correlated timely with neurotonic discharges in the left side X cranial nerve, bilateral LAR, and left vocal co-MEP decrement in amplitude response, alongside with significantly decreased HR and BP. Moreover, the left side X cranial nerve IONM disturbances improvement, HR/BP recovery, and surgical pause coincided. Hence, we believe that the plausible cause of the hemodynamic distress in our case was due to either lower brainstem, left side X cranial nerve, or both neural structures handling/stretching, epitomized as a vagal depressor reaction. We hypothesized that hemodynamic instability could have been most likely attributed to the mediated hyperirritability of vagal dorsal motor nucleus fibers due to constant volley of the afferent impulses. These impulses may have been transferred either directly to the vagal nucleus or through secondary afferent collateral from the nucleus of the tractus solitarius of the tenth nerve.[
Muscle vocal MEP combined with LAR as a technique for monitoring the entire vagal reflex arc provided useful intraoperative data, allowing for a feasible prediction of the clinical status in our patient. Our patient developed transient mild dysphagia immediately after surgery, proving a suitable correlation of intraoperative monitoring changes and postoperative vagal nerve status. Using this data, it is possible to change the surgical technique before causing permanent neurological harm. Without any doubt, IONM plays a major part in directing the surgeon and avoiding damage to the cranial nerves, the motor nuclei of the cranial nerves, and the brainstem during surgery.[
A combination of vocal cord CoMEP and LAR monitoring in surgeries where VN integrity is at risk may improve future troubleshooting algorithms in the event of unilateral/ bilateral loss or significant amplitude decrement of LAR responses. There are few documented cases where LAR monitoring has been used in posterior fossa surgeries, but there were no true positive results. Such results of the few published cases showed either preservation of LAR responses with good nerve functional outcome or permanent loss of unilateral LAR response with no postoperative neurological deficits.[
CONCLUSION
LAR is a novel method for assessing VN integrity in brainstem surgeries, in conjunction with other IONM modalities. As in our case, LAR shows consistent and constant monitoring of VN and preemptively avoids permanent and severe sequela. We advocate the use of LAR in brainstem and posterior fossa surgeries.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amano M, Kohno M, Nagata O, Taniguchi M, Sora S, Sato H. Intraoperative continuous monitoring of evoked facial nerve electromyograms in acoustic neuroma surgery. Acta Neurochir (Wien). 2011. 153: 1059-67
2. Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Modulation of laryngeal responses to superior laryngeal nerve stimulation by volitional swallowing in awake humans. J Neurophysiol. 2000. 83: 1264-72
3. Chikao N, Arata S, Akira K, Seiichi K. Cardiovascular complications on upper vagal rootlet section for glossopharyngeal neuralgia; case report. J Neurosurg. 1976. 44: 248-53
4. Costa P, Gaglini PP, Tavormina P, Ricci F, Peretta P. A method for intraoperative recording of the laryngeal adductor reflex during lower brainstem surgery in children. Clin Neurophysiol. 2018. 129: 2497-8
5. Henriquez VM, Schulz GM, Bielamowicz S, Ludlow CL. Laryngeal reflex responses are not modulated during human voice and respiratory tasks. J Physiol. 2007. 585: 779-89
6. Jahangiri F, Minhas M, Jane J. Preventing lower cranial nerve injuries during fourth ventricle tumor resection by utilizing intraoperative neurophysiological monitoring. Neurodiagn J. 2012. 52: 320-32
7. Misher ET, Smith P. Technical aspects of intraoperative monitoring of lower cranial nerve function. Skull Base Surg. 1995. 5: 245-50
8. Mochida A. Reflex control on laryngeal functions-vibration effect of the laryngeal mucosa on recurrent laryngeal nerve reflexes. Nihon Jibiinkoka Gakkai Kaiho. 1990. 93: 938-48
9. Sandler ML, Sims JR, Sinclair C, Sharif KF, Ho R, Yue LE. Vagal schwannomas of the head and neck: A comprehensive review and a novel approach to preserving vocal cord innervation and function. Head Neck. 2019. 41: 2450-66
10. Satomaa AL, Vanttinen S, Mattila H. The intraoperative laryngeal adductor reflex (LAR) in brainstem tumor removal: A case of unilateral loss of LAR signal. Clin Neurophysiol. 2019. 130: 1253-5
11. Sinclair CF, Tellez MJ, Tapia OR, Ulkatan S, Deletis V. A novel methodology for assessing laryngeal and vagus nerve integrity in patients under general anesthesia. Clin Neurophysiol. 2017. 128: 1399-405
12. Sinclair CF, Tellez MJ, Ulkatan S. Human laryngeal sensory receptor mapping illuminates the mechanisms of laryngeal adductor reflex control. Laryngoscope. 2018. 128: E365-70
13. Sinclair CF, Tellez MJ, Ulkatan S. Noninvasive, tube-based, continuous vagal nerve monitoring using the laryngeal adductor reflex: Feasibility study of 134 nerves at risk. Head Neck. 2018. 40: 2498-506
14. Tellez MJ, Ulkatan S, Blitzer A, Sinclair CF. Unearthing a consistent bilateral R1 component of the laryngeal adductor reflex in awake humans. Laryngoscope. 2018. 128: 2581-7
15. Ulkatan S, Tellez MJ, Sinclair C. Laryngeal adductor reflex and future projections for brainstem monitoring, Reply to a method for intraoperative recording of the laryngeal adductor reflex during lower brainstem surgery in children. Clin Neurophysiol. 2018. 129: 2499-500