- Department of Neurosurgery, Kawasaki Medical School, Kurashiki, Japan.
- Department of Okayama Kyokuto Hospital, Okayama, Japan.
Correspondence Address:
Shunji Matsubara, Department of Neurosurgery, Kawasaki Medical School, Kurashiki, Okayama, Japan.
DOI:10.25259/SNI_557_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shunji Matsubara1, Hiroyuki Toi1, Hiroki Takai1, Yuko Miyazaki1, Keita Kinoshita1, Yoshihiro Sunada1, Shodai Yamada1, Yoshifumi Tao1, Noriya Enomoto1, Yukari Ogawa Minami1, Satoshi Hirai1, Kenji Yagi1, Hiroyuki Nakashima2, Masaaki Uno1. Variations and management for patients with craniocervical junction arteriovenous fistulas: Comparison of dural, radicular, and epidural arteriovenous fistulas. 16-Aug-2021;12:411
How to cite this URL: Shunji Matsubara1, Hiroyuki Toi1, Hiroki Takai1, Yuko Miyazaki1, Keita Kinoshita1, Yoshihiro Sunada1, Shodai Yamada1, Yoshifumi Tao1, Noriya Enomoto1, Yukari Ogawa Minami1, Satoshi Hirai1, Kenji Yagi1, Hiroyuki Nakashima2, Masaaki Uno1. Variations and management for patients with craniocervical junction arteriovenous fistulas: Comparison of dural, radicular, and epidural arteriovenous fistulas. 16-Aug-2021;12:411. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11045
Abstract
Background: Craniocervical junction arteriovenous fistulas (CCJAVFs) are known to be rare, but variations and clinical behaviors remain controversial.
Methods: A total of 11 CCJAVF patients (M: F=9:2, age 54–77 years) were investigated. Based on the radiological and intraoperative findings, they were categorized into three types: dural AVF (DAVF), radicular AVF (RAVF), and epidural AVF (EDAVF).
Results: There were four symptomatic patients (subarachnoid hemorrhage in two, myelopathy in one, and tinnitus in one) and seven asymptomatic patients in whom coincidental CCJAVFs were discovered on imaging studies for other vascular diseases (arteriovenous malformation in one, intracranial DAVF in two, ruptured cerebral aneurysm in two, and carotid artery stenosis in two). Of these 11 patients, 2 (18.2%) had multiple CCJAVFs. Of 14 lesions, the diagnoses were DAVF in 5, RAVF in 3, and EDAVF in 6 (C1–C2 level ratio =5:0, 2:1, 3:3). Patients with DAVF/RAVF in four lesions with intradural venous reflux underwent surgery, although an RAVF remained in one lesion after embolization/radiation. Since all six EDAVFs, two DAVFs, and one RAVF had neither feeder aneurysms nor significant symptoms, no treatment was provided; of these nine lesions, one DAVF and one RAVF remained unchanged, whereas six EDAVFs showed spontaneous obliteration within a year. Unfortunately, however, one DAVF bled before elective surgery.
Conclusion: CCJAVFs have many variations of shunting site, angioarchitecture, and multiplicity, and they were frequently associated with coincidental vascular lesions. For symptomatic DAVF/RAVF lesions with intradural drainage, surgery is preferred, whereas asymptomatic EDAVFs without dangerous drainage may obliterate during their natural course.
Keywords: Craniocervical junction arteriovenous fistula, Dural, Epidural, Multiplicity, Radicular
INTRODUCTION
A craniocervical junction arteriovenous fistula (CCJAVF) is a rare and peculiar disorder causing subarachnoid hemorrhage (SAH), myelopathy, brain stem dysfunction, occipital neuralgia, or neck pain, although thoracolumbar spinal arteriovenous fistulas (AVFs) mainly affect men and usually cause slowly progressive myelopathy due to venous congestion.[
MATERIALS AND METHODS
A total of 11 CCJAVF patients (nine males and two females; age range 54–77 years; mean age 67.5 years) managed at our institution between July 2007 and March 2021 were studied. This cohort included four symptomatic patients and seven asymptomatic patients. Two patients had SAH, one had myelopathy, one had tinnitus, and seven had no symptoms because they were incidentally found during examinations for other disorders, including ruptured cerebellar arteriovenous malformation (AVM) in one, ruptured tentorial DAVF in one, symptomatic optic canal DAVF in one, ruptured cerebral aneurysms in two, and origin of internal carotid artery stenosis in two. All lesions were recognized at the C1 or C2 level. All patients underwent a variety of examinations including magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomography angiography (CTA), and digital subtraction angiography (DSA) to identify feeding arteries, the precise location of the shunt point, draining veins, and the presence of a feeder aneurysm or varix. After recognizing CCJAVFs, coronal sections of MRI sequences such as 3D fast advanced spin echo (3D FASE, Vantage Titan 3T, Canon Medical Systems, Tochigi, Japan), 3D fast spin echo (CUBE, 3T Signa Excite HDx, GE Medical, Chicago, USA), short Tl inversion recovery (STIR, Signa Excite Xl 1.5T, GE Medical), and fast imaging employing steady state acquisition (FIESTA, Signa Excite XL 1.5T, GE Medical) were additionally performed in five patients to clarify the fistula point in various surrounding anatomical structures.
Based on the currently proposed classification,[
As for treatment, microsurgery, transarterial embolization, or CyberKnife radiation therapy were considered depending on the symptoms and the patient’s general condition. Follow-up DSA was conducted to confirm the fistulous lesion in most patients.
RESULTS
Of the 11 patients, 2 (18.2%) had multiple CCJAVFs: one patient with 3 synchronous EDAVF lesions at the C1 and C2 levels, and one patient with 2 metachronous bilateral EDAVF lesions at the C2 level.
Of a total of 14 lesions, 5 (35.7%) were diagnosed as DAVFs, 3 (21.4%) were diagnosed as RAVFs, and the remaining 6 (42.9%) were diagnosed as EDAVFs [
With respect to angioarchitecture, in five DAVF lesions, preoperative angiography showed feeding arteries of the C1–C3 radiculomeningeal artery (RMA) in five and the external carotid artery (ECA) in one, whereas in three RAVF lesions, the C1–C3 RMA was involved in three lesions, the ECA in one lesion, and the anterior spinal artery (ASA) in two lesions. A tiny feeder aneurysm was found in one RAVF lesion, whereas a varix of the draining vein was found in one DAVF lesion. Of the six EDAVF lesions, they were supplied by the RMA in one, the ECA in one, the posterior meningeal artery in three, and the prelaminar artery in two. In all DAVF patients, the shunt point was identified in the caudal side of the dural penetration of the vertebral artery (VA) on an MRI coronal image or as an intraoperative finding. On the other hand, in RAVF patients, the shunt point was identified on the C1 nerve root on an MRI image in two patients and as an intraoperative finding in one patient. The draining vein was single in all patients in both groups. In DAVF patients, the draining vein ascended to the cranial side alone in one patient, descended to the spinal side in two, and was bidirectional through the radicular vein in two, whereas in RAVF patients, the draining vein ascended in one and was bidirectional in two. In EDAVF patients, three C1 lesions showed drainage to the suboccipital cavernous sinus, whereas three C2 lesions showed drainage to the epidural venous plexus.
Regarding management and outcomes, in the DAVF group, three patients underwent craniotomy, and drainer clipping was successfully conducted, whereas in the RAVF group, one patient underwent surgery, and one patient underwent coil embolization followed by CyberKnife therapy. No patient in either group had perioperative complications. In three DAVF patients who underwent microsurgery, the drainer was found to arise under the dural penetration of the VA and the C1 spinal nerve root. In one RAVF patient who underwent microsurgery, the drainer originated directly from the C1 nerve root that was between the dura and spinal cord. All three DAVF patients and one RAVF patient had postoperative angiography to confirm complete obliteration. However, one patient with RAVF had a remaining lesion 2 years after CyberKnife treatment. In the DAVF group, four patients experienced good recovery, but one patient had fatal bleeding 2 months before the elective surgery. In the RAVF group, two operative patients recovered fully, and one patient was unchanged. In three EDAVF patients with six lesions, no aggressive therapy was conducted due to insignificant symptoms. Nevertheless, follow-up angiography or MR angiography 1 year later confirmed complete obliteration. As for the outcomes, two patients had good recovery, but one patient became severely disabled due to primary damage from the aneurysmal SAH.
CASE PRESENTATION
Case 1 (Patient no. 5)
A 77-year-old man had suffered from motor weakness of the left upper limb and bilateral lower limbs for a year and visited our institution. Sagittal MRI (T2WI) showed a hyperintense signal in the upper cervical spinal cord [
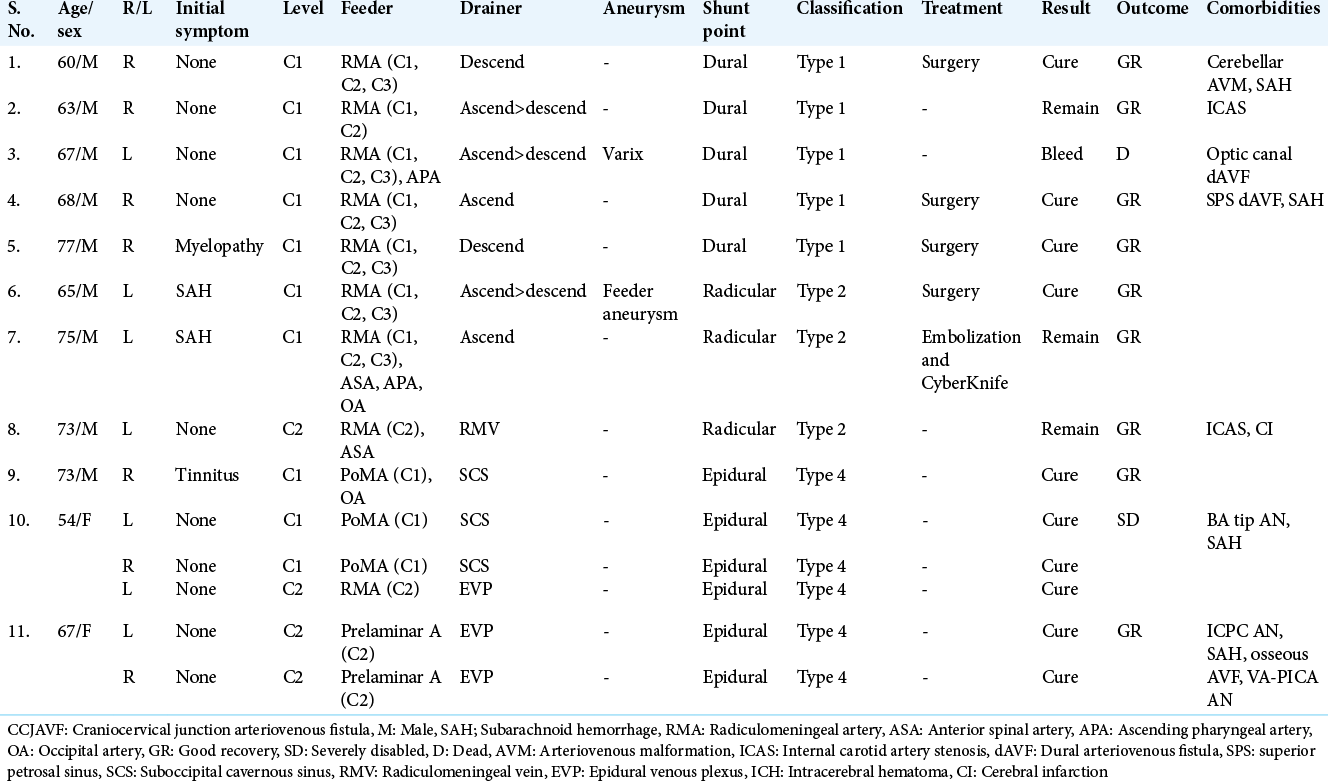
Figure 1:
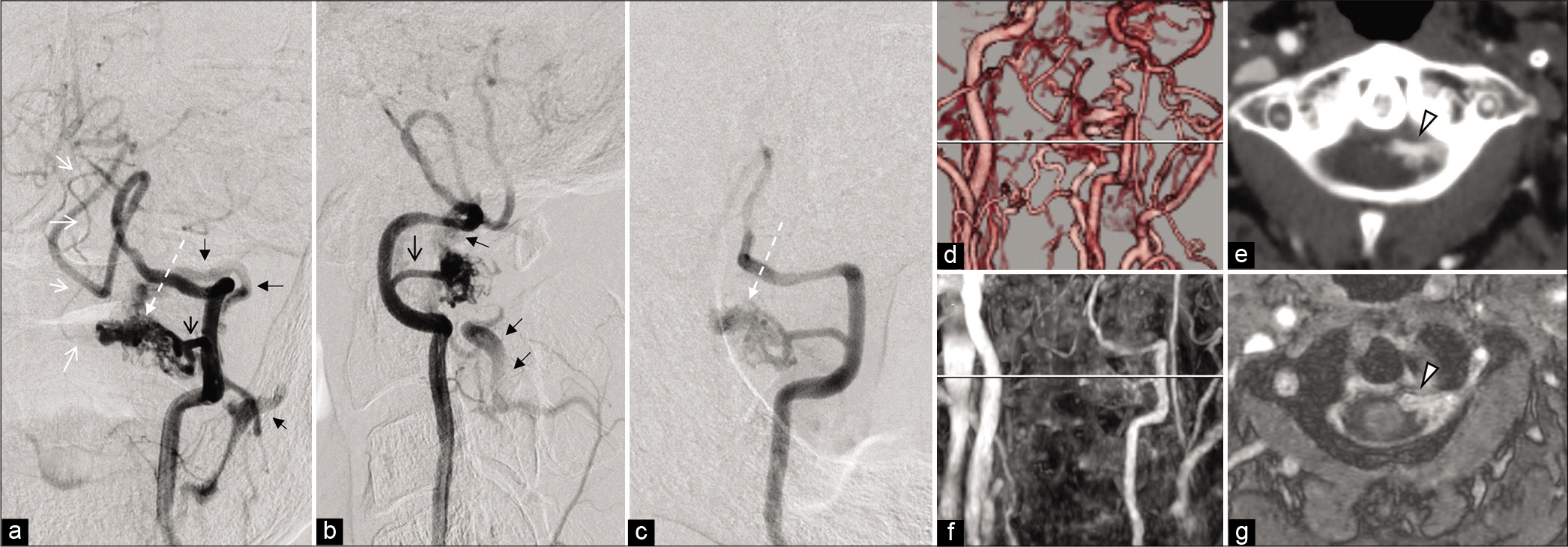
(Patient no. 5) (a) The sagittal section of T2WI shows a hyperintense area in the upper cervical cord and an abnormal flow void in the ventral side of the medulla oblongata. (b and c) Frontal view of the right VAG demonstrates an AV shunt at the foramen magnum level involving the RMAs of right C1–C3, with drainage by the radicular vein leading to the anterior medullary vein and anterior spinal vein (white arrowheads). (d) 3D FASE of MRI shows that the draining vein originates from below the VA dural penetration on the coronal view (dotted arrows). (e) In the prone position, the microscopic view demonstrates vessel structures that are relevant to this AV shunt, neurons, and a ligament. (f) Magnifying view confirms the shunt point where draining vein (double black arrows) seems to connect directly to the VA (white arrow head). (g) Schematic diagram of the operative view. (h) The main drainer is disconnected by clipping. RMA: Radiculomeningeal artery, VA: Vertebral artery.
Case 2 (Patient no. 6)
A 65-year-old man presented with SAH. Preoperative left VA injection demonstrated a CCJAVF on the left side that arose from the RMA of C1–3 with drainage to the radicular vein connecting to the ascending intracranial vein and descending spinal vein [
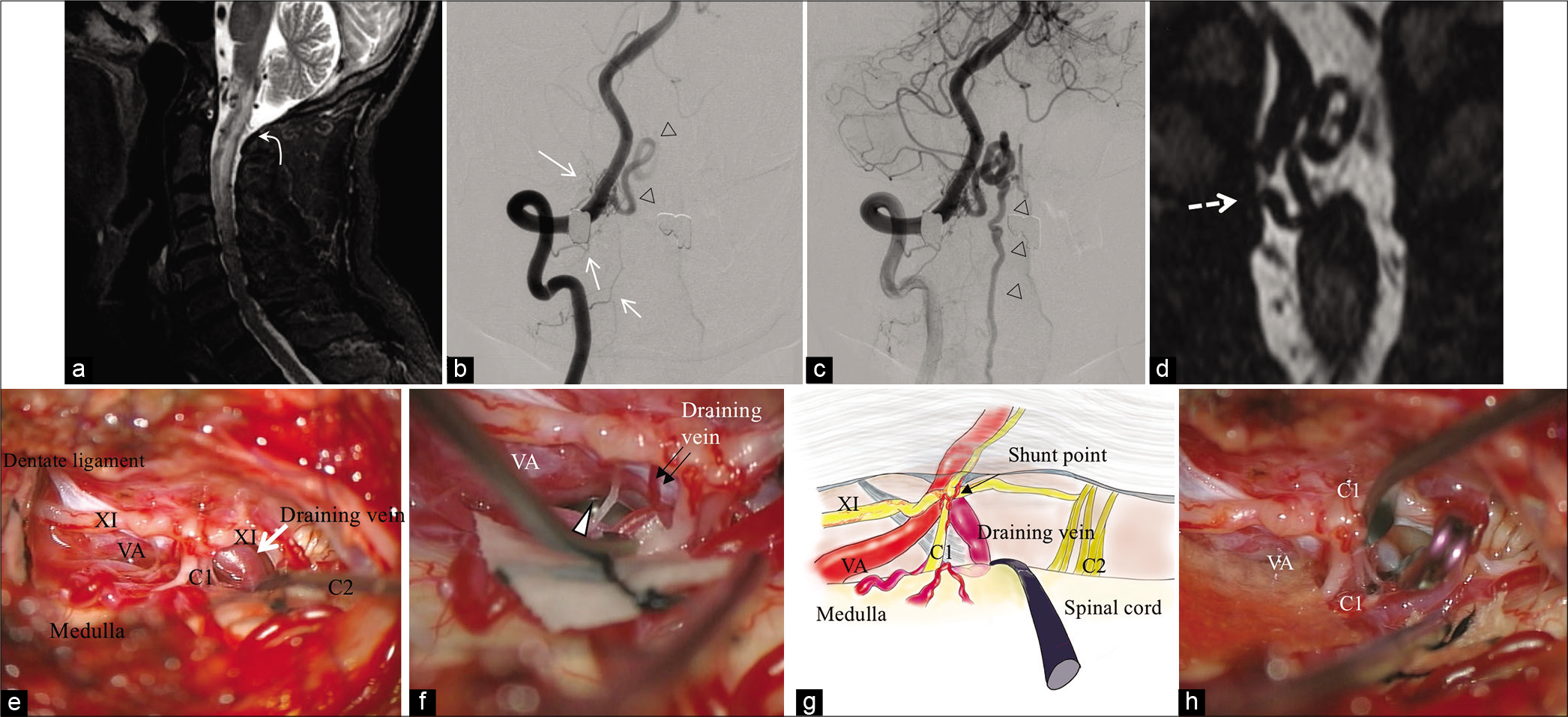
Figure 2:
(Patient no. 6). Left vertebral angiogram ([a] frontal view, [b] LAO51, [c] LAO45 cranial 27) indicates the abnormal AV fistulous point (white arrow) arising from the RMAs of C1-3 with single drainage to the ascending and descending veins. A tiny aneurysm on the feeding artery (C1-RMA) is recognized (black arrow). The oblique view showing that the gap between the VA and ostium of the drainer ([c] bidirectional arrow) is noted. The shunt point seems to be tangled with fine vessels ([b and c] asterisk). (d) Coronal MIP images of CTA also confirm the shunt point (white arrowhead). (e) Intraoperative view after cutting the dorsal root of the left C1 nerve and dentate ligament. The ventral root of C1 is involved with tangled small vessels from the VA side and medullary side (white arrowheads). The main drainer originates from the C1 ventral nerve roots (white long arrow), and the angioarchitecture is depicted with ICG (f). (g) The schematic diagram of the intraoperative view. Permanent occlusion is performed (h). VA: Vertebral artery, RMA: Radiculomeningeal artery, CTA: Computed tomography angiography, ICG: Indocyanine green.
Case 3 (Patient no. 11)
A 67-year-old woman was brought to our institution with an SAH due to an internal carotid-posterior communicating (IC-PC) artery aneurysm on the left side. Emergent partial coil embolization was successfully conducted on the same day. Follow-up left VA injection on day 10 incidentally showed an AV fistula outside the dural sac (asterisk) supplied by the left C2 prelaminar artery, with drainage to the epidural venous plexus [
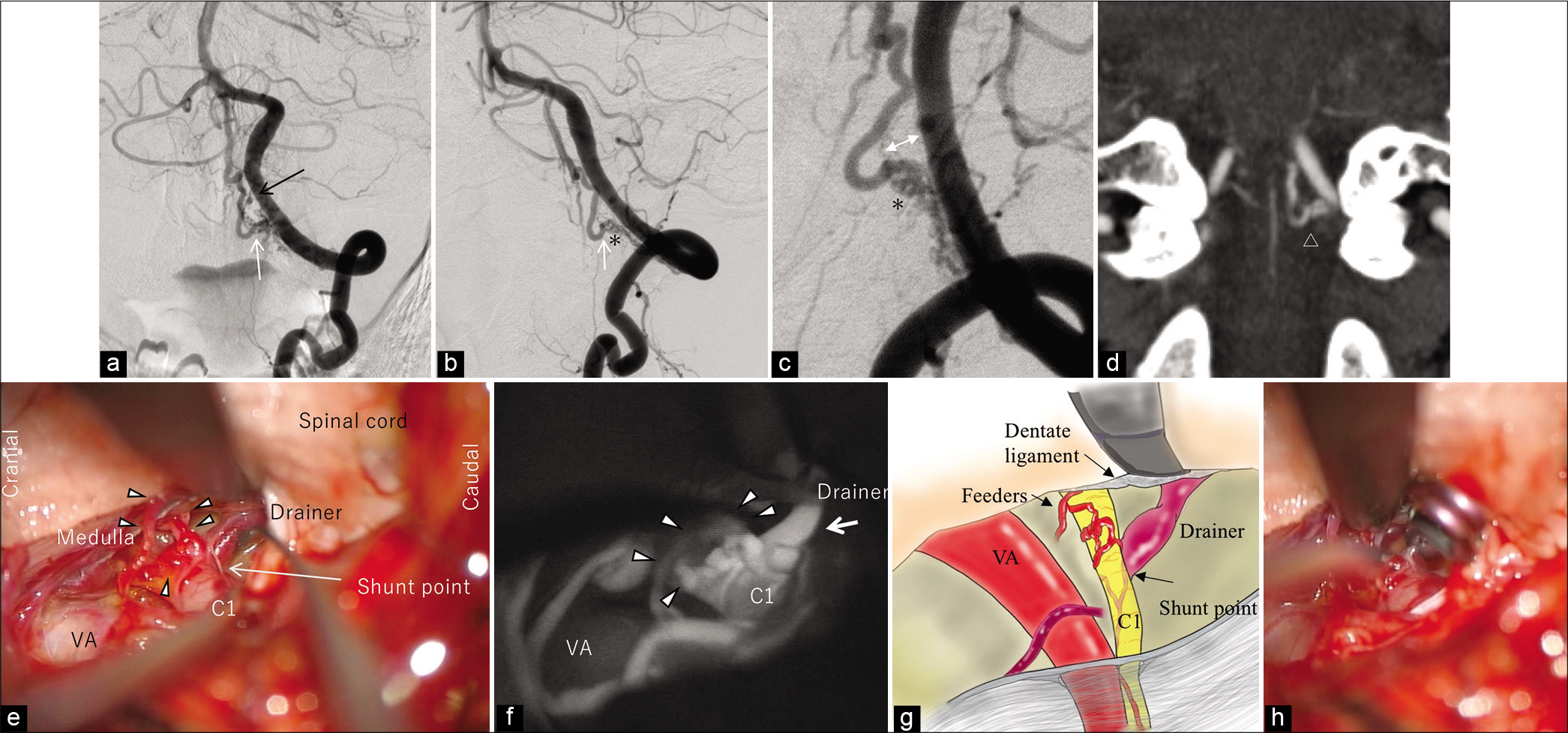
Figure 3:
(Patient no. 11). Left VA injection on day 10 indicates the AV fistula outside the dural sac (asterisk) supplied by the left prelaminar artery of C2 (long black arrows) with drainage to the epidural venous plexus ([a and b] frontal view, [c and d] lateral view). (e) Two months later, follow-up angiography shows the disappearance of the lesion. (f and g) The contralateral vertebral angiogram, however, demonstrates the de novo fistula (asterisk) at the C2 level supplied by the prelaminar artery (black arrow) with drainage to the venous plexus. (h) At this examination, the carotid artery angiogram also shows another AVF (possibly an osseous AVF) in the middle cranial base fed by the accessory meningeal artery (single white arrow) with drainage to the maxillary vein (double white arrows). (i) Two months after its occurrence, it has disappeared. (j) The angiogram obtained 6 months following formation also confirms complete obliteration of the CCJAVF on the right side. VA: Vertebral artery, AVF: Arteriovenous fistula, CCJAVF: Craniocervical junction arteriovenous fistula.
Case 4 (Patient no. 8)
A 73-year-old man with a history of old cerebral infarction, peripheral artery disease, diabetes mellitus, hypertension, and smoking had dizziness and visited our institution. His MRI demonstrated recurrence of cerebral infarction and severe internal carotid artery stenosis. On angiography, the left VA injection showed a CCJAVF consisting of nidus-like formation in the spinal canal at the C2 level arising from the left RMA and ASA with drainage to the radiculomeningeal vein of C1 and C2 leading to the epidural venous plexus [
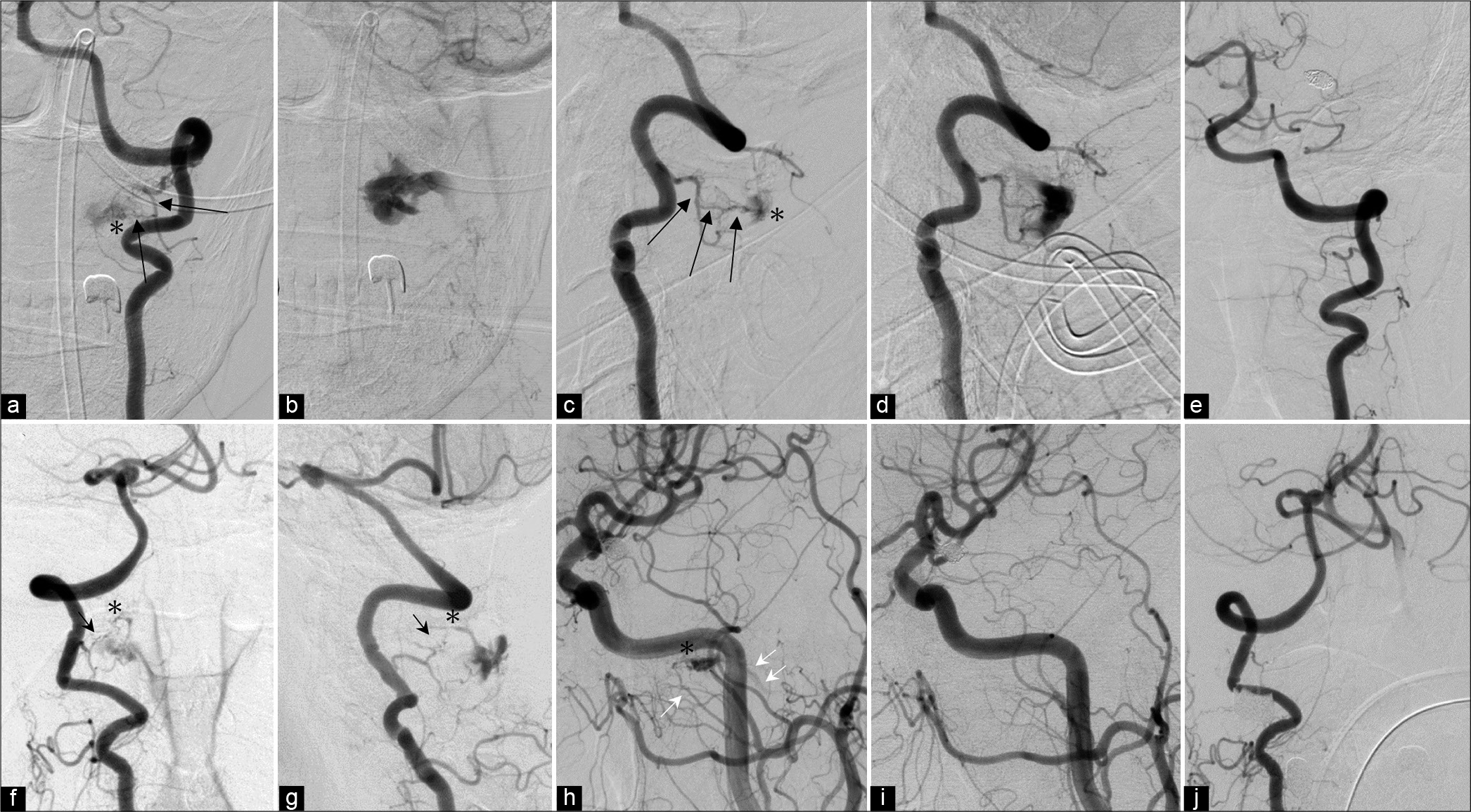
Figure 4:
(Patient no. 8). (a-c) (a: frontal view, b: lateral view, c: right oblique view): left vertebral artery injection shows the CCJAVF consisting of nidus-like formation in the spinal canal at the C2 level (white dotted arrow), arising from the RMA (black open arrow) and the ASA with drainage to the radiculomeningeal vein of C1 and C2 to the epidural venous plexus (black closed arrow). (d and e) CTA discloses the fistulous point on the anterior root of the C2 nerve (white arrow head). (f and g) Gd-DTPA MRA also shows that the abnormally enhancing lesion is mainly located on the anterior cervical nerve root inside the dura mater (white arrow head). RMA: Radiculomeningeal artery, ASA: Anterior spinal artery, CTA: Computed tomography angiography, MRA: Magnetic resonance angiography.
DISCUSSION
Classification
Because spinal AVMs have many variations, they can be difficult to categorize clearly. The classification by Anson and Spetzler, which is based on the anatomy and appearance, has been generally used and includes type I: DAVF, type II: glomus AVM, type III: juvenile AVM, and type IV: intradural perimedullary AVF.[
Although a CCJAVF may be regarded as being in the category of spinal AVF, it includes the element of intracranial DAVF due to the high incidence of intracranial bleeding. However, recent developments in various neuroradiological modalities and accumulation of surgical experience have led to identification of a variation of AVF that includes the combination type of perimedullary and DAVF,[
Differential diagnosis, clinical course, and management
In the present study, the diagnosis was DAVF in five patients, RAVF in three patients, and EDAVF in three patients. Clinical behavior and management should be determined depending on the correct differential diagnosis. An RAVF is thought to form a shunt on a nerve root supplied by both the RMA and a pial artery. A Japanese, multicenter, research study described a total of 59 CCJAVF patients, including 37% with DAVF, 29% with RAVF, 24% with EDAVF, and 10% with perimedullary AVF; the study suggested an unexpectedly high prevalence of RAVF among all types. Moreover, the bleeding risk from RAVFs was 1.73-fold that from DAVFs.[
Regarding the two differential preoperative diagnoses, an RAVF is usually supplied by the ASA or lateral spinal artery, but involvement of these arteries is thought to be rare in DAVFs. Because these lesions, however, are very small and have complicated angioarchitecture, judgment is not always easy. In fact, one of our three RAVF lesions failed to show a faint pial supply on the preoperative angiogram, although the microscopic view during the operation identified the pial supply (patient no. 6). Fusion imaging with DSA and MRI might reinforce the differential diagnosis for these two types. If the fusion image, however, is not available, an oblique view on a vertebral angiogram should be taken to identify the gap showing a short distance between the fistulous point and the medial aspect of the ipsilateral VA. Both RAVFs in patients were found to have this finding, but no DAVF patients did. This finding may be useful to suggest the differential diagnosis of RAVF or DAVF at the C1 level on a routine angiogram.
On the other hand, EDAVFs mainly affect the lumbar level. They typically form a shunt in the narrow space between the ventral dura and the dorsal vertebral body, arising mostly from the dorsal somatic branch of a segmental artery, with drainage to a radiculomeningeal vein.[
Thinking of the gravity effect, intradural venous reflux arising from an EDAVF hardly occurs in the upper cervical region because the AVF is located at a higher level than the heart level in daily life, even after the formation of the AVF. That may be associated with the fact that all six EDAVFs never had intradural venous reflux, but EDAVFs in the lumbar spine often had reflux. Given this background, we would like to recommend aggressive treatment when CCJ-EDAVF patients have intradural drainage, marked venous congestion, or a concomitant pial feeder aneurysm.[
Multiplicity and etiology
It is said that venous hypertension may contribute to the genesis of these AVFs. As described above, venous pressure in the upper cervical level should be lower because of gravity in the daytime. However, a bed-ridden condition for a long time resulting from SAH with high intracranial pressure is assumed to lead to an increase in paravertebral venous pressure, thereby forming de novo AVFs. In fact, 4 of 11 patients with 7 of 14 lesions were incidentally found during examination for SAH relevant to a cerebral aneurysm or intracranial AV shunt.
Regarding comorbidities, the present cohort included five patients with various other AV shunt diseases, including a cerebellar AVM in one, intracranial DAVFs in two, and multiple CCJAVFs in two. The high incidence of multiple AV shunts may be attributed to venous hypertension affected by the formation of the primary AVM or other DAVF. Several authors documented the heterogeneous multiplicity, such as a CCJAVF with a soft-tissue AVF in the same metamere,[
In addition, minor trauma may be associated with the formation of AVFs. There is a special structure between the C1 and the C2 vertebra with the atlanto-axial joint facilitating the movement of wide neck rotation. It may affect the spinal nerve or VA due to repeated minor trauma in daily life, exercise, or sports, especially at the C1/C2 level, resulting in the genesis of a micro-AV shunt. This mechanism could be relevant to the frequency of de novo AVFs at the C1–C2 level, whereas it is less frequent at the thoracic spine level.
Development of RAVF and neurovascular wiring
However, the question of how we explain the hypothesis in which the spinal nerve plays a role in navigating an AVF must be considered. According to the literature, the neuronal network and blood vessel network interact with each other and are termed “neurovascular wiring,” “neurovascular link,” or “neurovascular congruence.”[
These new findings from academic research seem to be consistent with the theory that an AVF occurs adjacent to the nerve root at the CCJ level, as previously documented in a domestic, multicenter study.[
Study limitations
The present study has several limitations. First, the study design was a single-center, retrospective approach. Second, only 11 patients managed at our institution during the 14-year period were analyzed. The total number of patients was not enough to demonstrate the validity of our theory. Third, surgical confirmation of the shunt point was not performed in all patients. This rare, unique, and angiographically complicated disease requires a multicenter, prospective study to carefully establish its etiology and the optimal management.
CONCLUSION
CCJAVFs have many variations, such as in shunting site, angioarchitecture, and multiplicity, and they were frequently associated with coincidental vascular lesions. For symptomatic DAVF and RAVF lesions with intradural drainage, surgical disconnection is preferred, whereas asymptomatic EDAVFs without dangerous drainage may regress spontaneously on serial imaging.
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anson JA, Spetzler RF. Classification of spinal arteriovenous malformations and implications for treatment. Barrow Neurol Inst Q. 1992. 8: 2-8
2. Arai N, Akiyama T, Yoshida K. The coexistence of extradural arteriovenous fistula and soft tissue arteriovenous malformation within the same metamere. World Neurosurg. 2017. 98: 877.e1-7
3. Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005. 436: 193-200
4. Hiramatsu M, Sugiu K, Ishiguro T, Kiyosue H, Sato K, Takai K. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: A multicenter cohort study of 54 patients. J Neurosurg. 2018. 128: 1839-49
5. Houser OW, Campbell JK, Campbell RJ, Sundt TM. Arteriovenous malformation affecting the transverse dural venous sinus-an acquired lesion. Mayo Clin Proc. 1979. 54: 651-61
6. Jeon JP, Cho YD, Kim CH, Han MH. Complex spinal arteriovenous fistula of the craniocervical junction with pial and dural shunts combined with contralateral dural arteriovenous fistula. Interv Neuroradiol. 2015. 21: 733-7
7. Kanemaru K, Yoshioka H, Hashimoto K, Murayama H, Ogiwara M, Yagi T. Efficacy of intraarterial fluorescence video angiography in surgery for dural and perimedullary arteriovenous fistula at craniocervical junction. World Neurosurg. 2019. 126: e573-9
8. Kim DJ, Willinsky R, Geibprasert S, Krings T, Wallace C, Gentili F. Angiographic characteristics and treatment of cervical spinal dural arteriovenous shunts. Am J Neuroradiol. 2010. 31: 1512-5
9. Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998. 89: 755-61
10. Kiyosue H, Matsumaru Y, Niimi Y, Takai K, Ishiguro T, Hiramatsu M. Angiographic and clinical characteristics of thoracolumbar spinal epidural and dural arteriovenous fistulas. Stroke. 2017. 48: 3215-22
11. Kulwin C, Bohnstedt BN, Scott JA, Cohen-Gadol A. Dural arteriovenous fistulas presenting with brainstem dysfunction: Diagnosis and surgical treatment. Neurosurg Focus. 2012. 32: E10
12. Kuwayama N, Kubo M, Endo S, Sakai N. Present status in the treatment of dural arteriovenous fistulas in Japan. Jpn J Neurosurg (Tokyo). 2011. 20: 12-9
13. Lai CW, Agid R, van den Berg R, Ter Brugge K. Cerebral arteriovenous fistulas induced by dural arteriovenous shunts. AJNR Am J Neuroradiol. 2005. 26: 1259-62
14. Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interv Neuroradiol. 1997. 3: 275-81
15. Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997. 87: 267-74
16. Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG. Peripheral nerve-derived CXCL12 and VEGF-a regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013. 24: 359-71
17. Matsubara S, Satoh K, Satomi J, Shigekiyo T, Kinouchi T, Miyake H. Acquired pial and dural arteriovenous fistulae following superior sagittal sinus thrombosis in patients with protein S deficiency: A report of two cases. Neurol Med Chir (Tokyo). 2014. 54: 245-52
18. Miyata K, Iihoshi S, Koyanagi I, Wanibuchi M, Sugino T, Nomura K. Endovascular therapy of radicular arteriovenous fistula at the craniocervical junction fed by the posterior inferior cerebellar artery. J Neuroendovasc Ther. 2017. 11: 88-93
19. Mukouyama Y, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002. 109: 693-705
20. Niimi Y, Berenstein A, Fernandez PM, Brisman JL, Song JK. Pediatric nonvertebral paraspinal arteriovenous fistulas along the segmental nerve: Clinical, imaging, and therapeutic considerations. J Neurosurg. 2005. 103: 156-62
21. Nishio A, Ohata K, Takami T, Gotoh N, Tsuyuguchi N, Ichinose T. Spinal arteriovenous malformation associated with a radicular arteriovenous fistula suggested a metameric disease. A case report. Interv Neuroradiol. 2003. 9: 75-8
22. Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014. 159: 584-96
23. Oshita J, Yamaguchi S, Ohba S, Kurisu K. Mirror-image spinal dural arteriovenous fistulas at the craniocervical junction: Case report and review of the literature. Neurosurgery. 2011. 69: E1166-71
24. Phatouros CC, Halbach VV, Dowd CF, Lempert TE, Malek AM, Meyers PM. Acquired pial arteriovenous fistula following cerebral vein thrombosis. Stroke. 1999. 30: 2487-90
25. Puri AS, Telischak NA, Vissapragada R, Thomas AJ. Analysis of venous drainage in three patients with extradural spinal arteriovenous fistulae at the craniovertebral junction with potentially benign implication. J Neurointerv Surg. 2014. 6: 150-5
26. Rodesch G, Lasjaunias P. Spinal cord arteriovenous shunts: From imaging to management. Eur J Radiol. 2003. 46: 221-32
27. Sanders JB, O’Malley CD.editors. A discussion of the plates, and a biographical sketch of Vesalius with annotations and translations. The Illustrations from the Works of Andreas Vesalius of Brussels. New York: Dover Publications Inc; 1973. p. 131-53
28. Sato H, Wada H, Noro S, Saga T, Kamada K. Subarachnoid hemorrhage with concurrent dural and perimedullary arteriovenous fistulas at craniocervical junction: Case report and literature review. World Neurosurg. 2019. 127: 331-4
29. Sato K, Endo T, Niizuma K, Fujimura M, Inoue T, Shimizu H. Concurrent dural and perimedullary arteriovenous fistulas at the craniocervical junction: Case series with special reference to angioarchitecture. J Neurosurg. 2013. 118: 451-9
30. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992. 359: 843-5
31. Sorenson TJ, de Maria L, Rangel-Castilla L, Lanzino G. Surgical treatment of previously embolized craniocervical junction dural arteriovenous fistula. Neurosurg Focus. 2019. 46: V2
32. Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg. 2002. 96: 145-56
33. Suda S, Katsura K, Okubo S, Abe A, Kanamaru T, Ueda M. A case of dural arteriovenous fistulas at the craniocervical junction presenting with occipital/neck pain associated with sleep. Intern Med. 2012. 51: 925-8
34. Takahashi H, Ueshima T, Goto D, Kimura T, Yuki N, Inoue Y. Acute tetraparesis with respiratory failure after steroid administration in a patient with a dural arteriovenous fistula at the craniocervical junction. Intern Med. 2018. 57: 591-4
35. Takai K, Komori T, Kurita H, Kawai K, Inoue T, Taniguchi M. Intradural radicular arteriovenous fistula that mimics dural arteriovenous fistula: Report of three cases. Neuroradiology. 2019. 61: 1203-8
36. Takai K, Taniguchi M. Comparative analysis of spinal extradural arteriovenous drainage: A systematic literature review. Neurosurg Focus. 2012. 32: E8
37. Terada T, Higashida RT, Halbach VV, Dowd CF, Tsuura M, Komai N. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994. 80: 884-9
38. Terada T, Tsuura M, Komai N, Higashida RT, Halbach VV, Dowd CF. The role of angiogenic factor bFGF in the development of dural AVFs. Acta Neurochir (Wien). 1996. 138: 877-83
39. Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999. 91: 781-6
40. Wang JY, Molenda J, Bydon A, Colby GP, Coon AL, Tamargo RJ. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci. 2015. 22: 1701-7
41. Wang XC, Du YY, Tan Y, Qin JB, Wang L, Wu XF. Brainstem congestion due to dural arteriovenous fistula at the craniocervical junction: Case report and review of the literature. World Neurosurg. 2018. 118: 181-7
42. Wälchli T, Wacker A, Frei K, Regli L, Schwab ME, Hoerstrup SP. Wiring the vascular network with neural cues: A CNS perspective. Neuron. 2015. 87: 271-96
43. Wiszniak SE, Schwarz QP, Trainor PA.editors. Neural crest cells in vascular development. Neural Crest Cells Evolution. Development and Disease. San Diego, London: Elsevier; 2014. p. 313-33
44. Yoshida K, Sato S, Inoue T, Ryu B, Shima S, Mochizuki T. Transvenous embolization for craniocervical junction epidural arteriovenous fistula with a pial feeder aneurysm. Interv Neuroradiol. 2020. 26: 170-7
45. Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: A systematic review. J Neurointerv Surg. 2016. 8: 648-53
46. Zhong W, Zhang J, Shen J, Su W, Wang D, Zhang P. Dural arteriovenous fistulas at the craniocervical junction: A series case report. World Neurosurg. 2019. 122: e700-12