- 3rd Department (vascular pathology), Federal State Autonomous Institution “N. N. Burdenko National Medical Research Center of Neurosurgery” of the Ministry of Health of the Russian Federation,
- Department of Internal Diseases, Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation, Moscow, Russia.
Correspondence Address:
Yuri Pilipenko
3rd Department (vascular pathology), Federal State Autonomous Institution “N. N. Burdenko National Medical Research Center of Neurosurgery” of the Ministry of Health of the Russian Federation,
DOI:10.25259/SNI_326_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuri Pilipenko, Shalva Eliava, Dmitry Okishev, Elena Okisheva, Andronikos Spyrou. Vertebral artery and posterior inferior cerebellar artery aneurysms: Results of microsurgical treatment of eighty patients. 22-Nov-2019;10:227
How to cite this URL: Yuri Pilipenko, Shalva Eliava, Dmitry Okishev, Elena Okisheva, Andronikos Spyrou. Vertebral artery and posterior inferior cerebellar artery aneurysms: Results of microsurgical treatment of eighty patients. 22-Nov-2019;10:227. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9771
Abstract
Background: The choice of surgical approaches and options for the microsurgical vertebral artery (VA) and posterior inferior cerebellar artery (PICA) aneurysms repair remains controversial.
Methods: A retrospective analysis of the clinical, surgical, and angiographic data of 80 patients with VA and PICA aneurysms treated from 2012 to 2018 was performed.
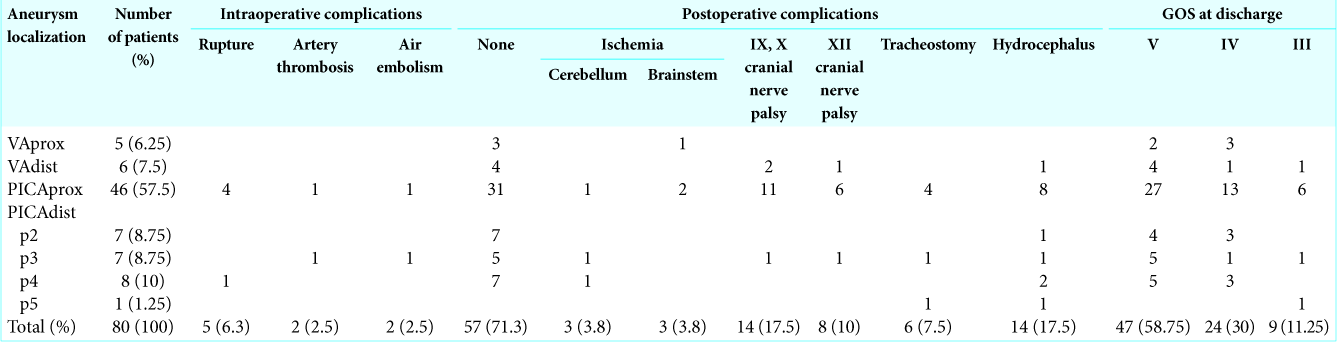
Results: The aneurysms were saccular in 50 cases (62.5%) and fusiform in 30 cases (37.5%). The median suboccipital craniotomy was the most common approach (73.8%). Retrosigmoid craniotomy was performed in 25% of patients. There were the following types of microsurgical operations: neck clipping (61.25%), clipping with the artery lumen formation (13.75%), trapping (10%), proximal clipping (5%), and deconstruction with anastomosis (10%). Fifty-seven (71.3%) patients were discharged without worsening of the clinical signs after surgery. The most common postoperative neurological disorder was palsy of IX and X cranial nerve revealed in 14 (17.5%) patients. No fatal outcomes or patients in vegetative state were identified. The complete occlusion of PICA and VA aneurysms according angiography was in 77 (96.3%) cases.
Conclusion: Microsurgical treatment is an effective method for VA and PICA aneurysms. The majority of VA and PICA aneurysms do not require complex basal approaches. A thorough preoperative planning, reconstructive clipping techniques, and anastomoses creation, as well as patient selection based on the established algorithms and consultations with endovascular surgeons, may reduce the number of complications and increase the rate of complete microsurgical occlusion in VA and PICA aneurysms.
Keywords: Anastomosis, Aneurysms, Bypass, Clipping, Median suboccipital craniotomy, Posterior inferior cerebellar artery, Retrosigmoid craniotomy, Vertebral artery
INTRODUCTION
The incidence of the aneurysms of the vertebral artery (VA) and posterior inferior cerebellar artery (PICA) is 2–4.5% of all brain aneurysms.[
The choice of treatment for VA and PICA aneurysms (microsurgical or endovascular surgery) remains controversial.[
This manuscript presents the results of the microsurgical treatment of VA and PICA aneurysms, types of surgical approaches, and options for aneurysm repair according to location and anatomical features.
MATERIALS AND METHODS
A retrospective analysis of the clinical, surgical, and angiographic data of sequential series of patients with VA and PICA aneurysms treated in the N. N. Burdenko National Medical Research Center of Neurosurgery from 2012 to 2018 was performed.
During this period, a total of 4106 patients with intracranial aneurysms underwent surgery in the Center of Neurosurgery. Among them, 130 (3.2%) patients had VA or PICA aneurysms. This group did not include 17 patients who had a combination of aneurysms with arteriovenous malformations of the posterior cranial fossa, as this group of patients had different management and outcomes.
The choice of the surgery type was based on the institutional algorithms for cerebral aneurysms,[ Preference for endovascular surgery in patients with aneurysms of the intracranial VA segment Preference for microsurgery for all PICA aneurysms and VA aneurysms with PICA originating from the aneurysm neck or sac.
Contraindications for endovascular surgery included
The acute subarachnoid hemorrhage (SAH) requiring stenting and antiplatelet therapy Resistance to or intolerance of the antiplatelet therapy Limited endovascular approach to the aneurysm (VA kinking and atherosclerosis).
In patients with contraindications to endovascular surgery or with unsuccessful endovascular attempts, microsurgery is recommended.
Contraindications for microsurgical treatment included severe decompensated medical conditions and systemic hypocoagulation.
A total of 80 microsurgical operations and 57 endovascular operations were performed. The outcomes of endovascular surgery are out of the scope of this manuscript.
Clinical characteristics of patients in the study group
Among all patients, 29 (36.3%) were males, and 51 (63.8%) were females. The age of patients ranged from 7 to 68 years (mean, 46.4 years).
A history of SAH was reported in the majority of patients – 75 (93.8%); however, only 12 patients underwent surgery within the first 14 days.
Five patients (6.3%) had no history of hemorrhage: three patients had asymptomatic aneurysms that were incidental findings, and in two patients with giant aneurysms signs of the disease were associated with an increase of the mass effect.
Eight patients (10%) had multiple aneurysms. PICA aneurysms caused hemorrhage in 5 of 7 cases when combined with aneurysms of the anterior circulation.
Anatomical and topographical characteristics of aneurysms in the study group
The intracranial part of the VA can be divided into the three segments: VA proximal to the PICA (VAprox); VA in the area of the PICA origin; and VA distal to the PICA (VAdist).
In accordance with the classification of Lister et al.,[
Peerless and Drake classified all PICA as proximal (about 1 cm from the PICA origin) and distal.[
Thus, we identified four main segments of VA and PICA aneurysm localization: (1) VAprox; (2) VAdist; (3) PICAprox; and (4) PICAdist.
Surgical approaches in the study group
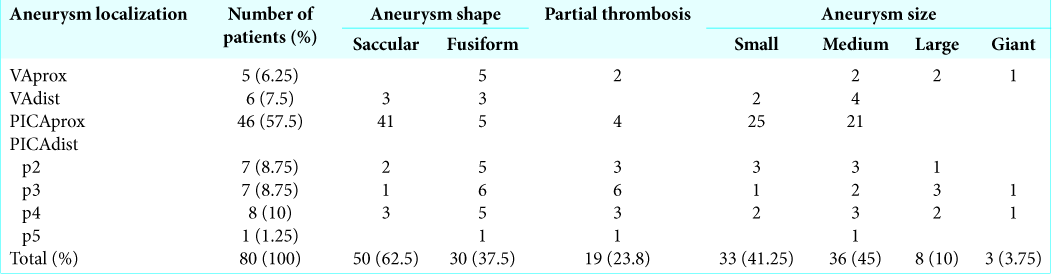
Three types of approaches [
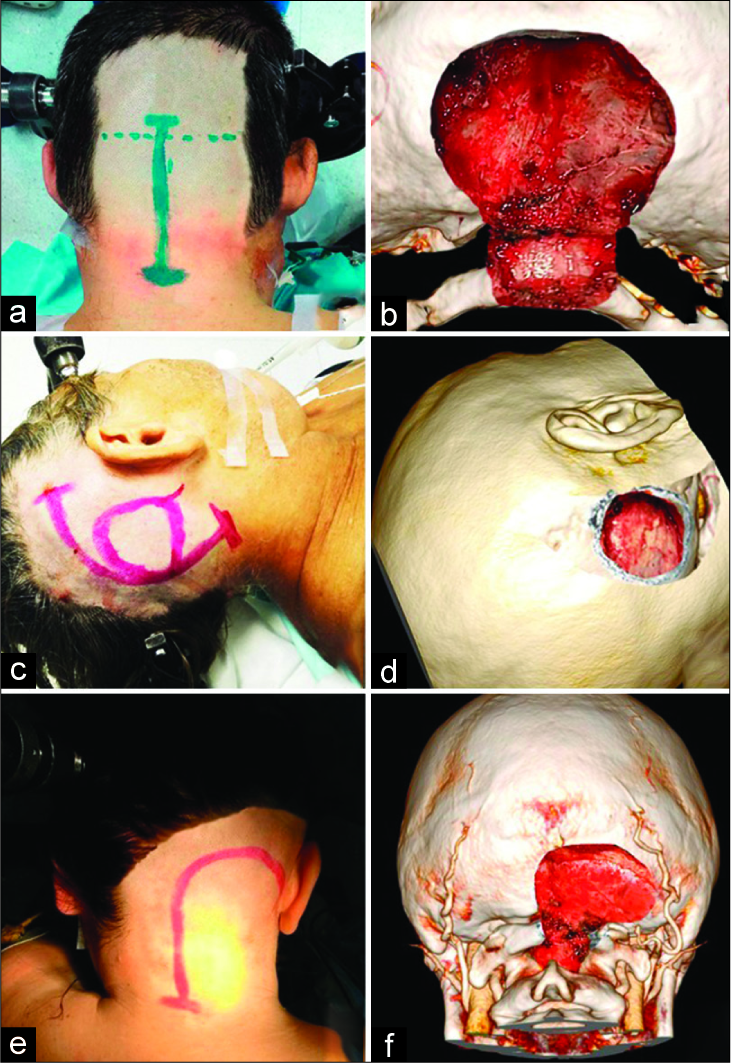
Figure 2:
Positions of patients on the operating table. Sitting position (a): can be used for all three approaches; Supine position with contralateral head and operating table rotation (b): is used for retrosigmoid approach; Prone position (c): is used for suboccipital approach; Lateral position (d): is used for far-lateral transcondylar approach.
SURGICAL RESULTS
The median suboccipital craniotomy (MSC) with lateralization toward the cerebellum hemisphere according to the location of the aneurysm was the most common approach (73.8%) [
The posterior arch of the cervical vertebrae was also resected through MSC in 40.7% [
In 47 patients, the MSC was performed in the sitting position. In 12 cases, where the aneurysm repair with anastomosis creation was considered before the surgery, the MSC was performed in the prone position.
Far-lateral transcondylar approach with resection of the posterior arch of the atlas was performed only in one case with the VAdist aneurysm close to the VA confluence. The approach was made in the lateral position from a hockey stick incision [
Retrosigmoid craniotomy from a linear or arcuate skin incision was performed in 25% of patients [
The choice of the aneurysm surgery [
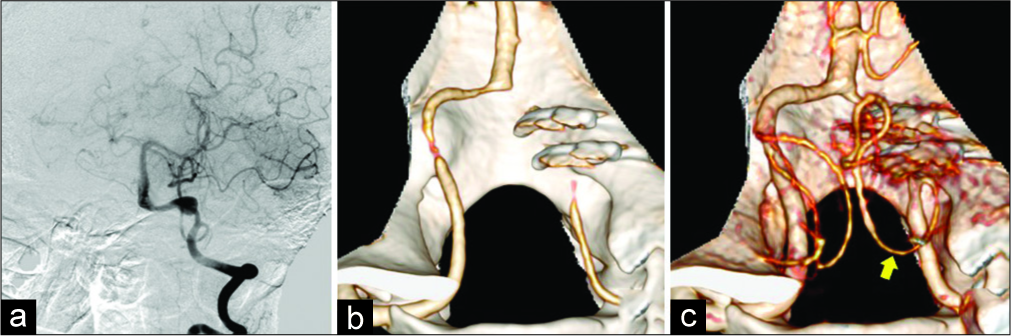
In saccular aneurysms, the clipping of the aneurysm neck was performed in the vast majority of patients (49 [98%] of 50) regardless of location. This type of aneurysm surgery was more common in the PICAprox aneurysms [
In the fusiform aneurysms, complex clipping with the artery lumen formation (CALF) was relatively more common. This operation was aimed at the clipping of the eccentric part of the fusiform aneurysm sparing the blood flow in the parent artery [
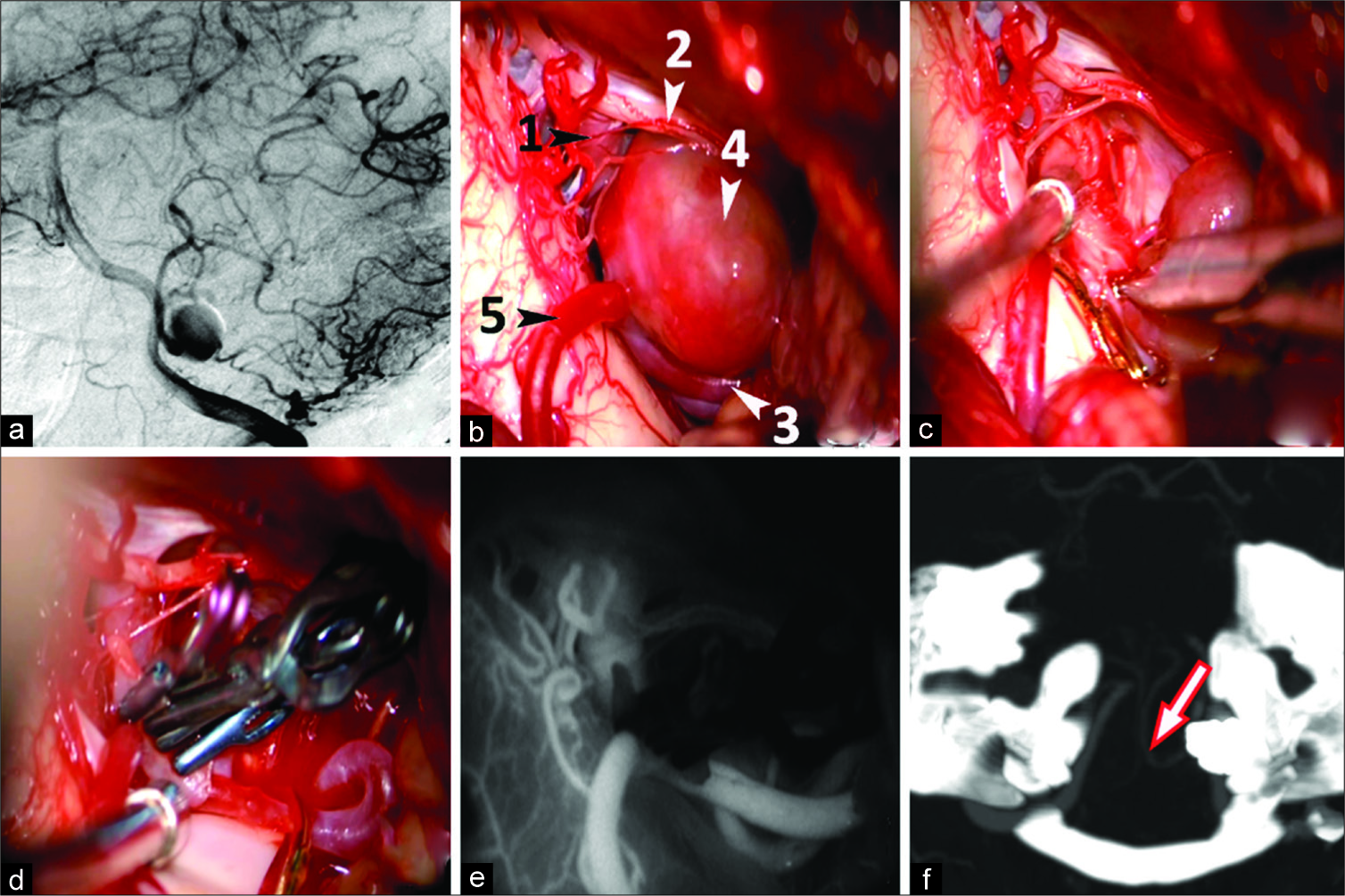
Figure 3:
Clipping with artery lumen formation for the eccentric-fusiform partially thrombosed aneurysm of the tonsillomedullary (p3) segment of the left PICA in a 35-year-old female patient. Vertebral angiography, lateral view (a): the functional part of the left PICA aneurysm is enhanced; Surgical view before clipping (b): 1 - proximal intracranial segments of the left VA, 2 - XI cranial nerve, 3 - PICA proximal to the aneurysm, 4 - aneurysm, 5 - PICA distal to the aneurysm; excision of the eccentric thrombosed part of the aneurysm (c); formation of the artery lumen via transverse applying of 4 clips (d); confirmation of PICA patency after clipping via ICG angiography (e); Post-op CT angiography on day 7: the arrow indicates patent left PICA (f).
Only in one of 4 cases with partially thrombosed saccular aneurysms, preoperative thrombectomy from the aneurysm cavity was required for the neck clipping.
In fusiform partially thrombosed aneurysms (n = 15), preliminary thrombectomy before CALF was required in 2 cases with PICAdist aneurysms. In 8 cases of large and giant partially thrombosed PICAdist aneurysms, thrombectomy was performed after aneurysm clipping for decompression of the adjacent brain and nerve structures. In 5 cases with small fusiform aneurysms, thrombectomy was not performed.
Deconstructive surgery (proximal clipping or trapping) was performed in 12 fusiform aneurysms [
The decision about aneurysm occlusion with the PICA segment was made after confirmation of the satisfactory retrograde blood flow through the distal PICA based on the intraoperative video angiography with indocyanine green (ICG) [
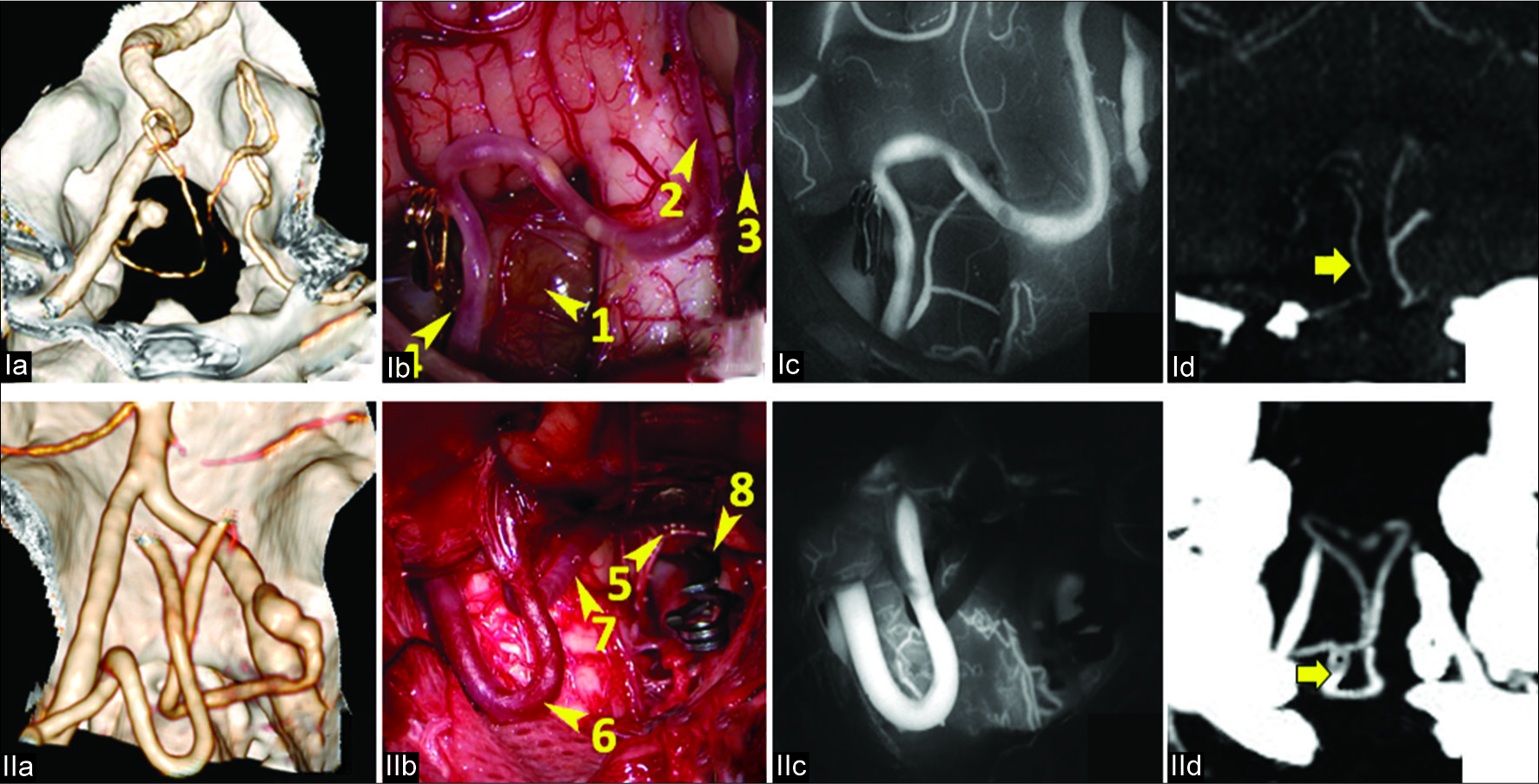
Figure 4:
The occlusion test with fluorescein video angiography. I. Patient V., 40 years. Ia. CT-angiography (3D reconstruction): PICAprox fusiform aneurysm on the left; Ib. Surgical view: 1. Aneurysm. 2. Left PICA. 3. Right PICA. 4. Temporary clip on the origin of the left PICA; Ic. ICG video angiography: satisfactory retrograde enhancement of the left PICA indicating good collateral blood flow; Id. CT angiography (MIP) after the aneurysm trapping: retrograde contrast in the left PICA (arrow). II. Patient P., 49 years. IIa. CT-angiography (3D reconstruction): PICAprox fusiform aneurysm on the right; IIb. Surgical view: 5. Aneurysm, 6. Left PICA. 7. Right PICA. 8. Clip on the origin of the right PICA; IIc. ICG video angiography: no retrograde enhancement of the right PICA indicating a lack of collateral blood flow; IId. CT angiography (MIP) after proximal aneurysm clipping followed by the reimplantation of the right PICA into the left PICA: anastomosis is indicated by the arrow.
Other criteria when PICA deconstruction should be combined with anastomosis may include: large diameter of the involved PICA, hypoplasia of the contralateral PICA or VA, as well as poor enhancement of the ipsilateral PICA and SCA according to angiography.
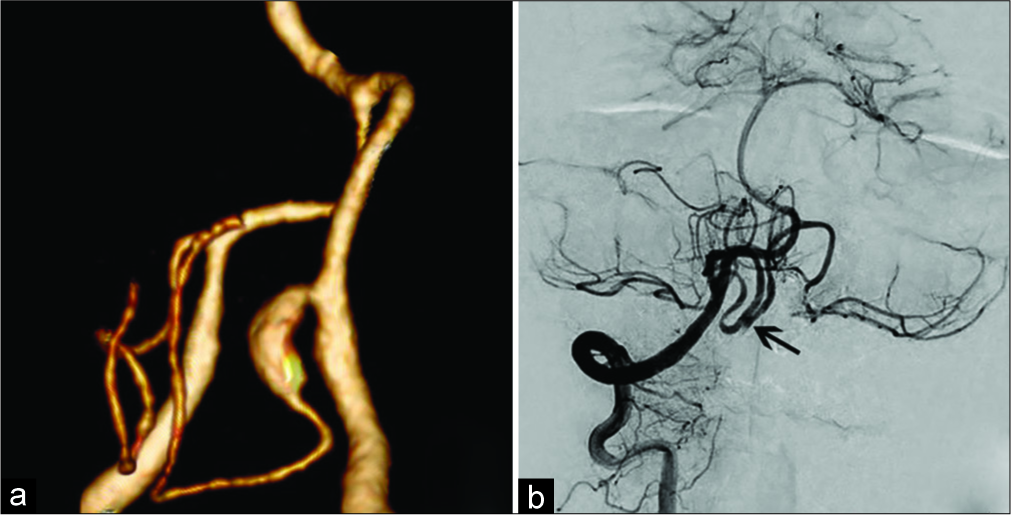
In most cases (6 of 8), revascularization involved local (in situ) anastomoses: “side-to-side” in one patient [
Figure 5:
“Side-to-side” anastomosis in a 52-year-old patient with PICAprox fusiform aneurysm. a. CT-angiography (3D reconstruction): fusiform aneurysm of the right PICAprox; b. Left vertebral angiography after trapping of the right PICAprox aneurysm and anastomosis creation (arrow) between the right and left PICA.
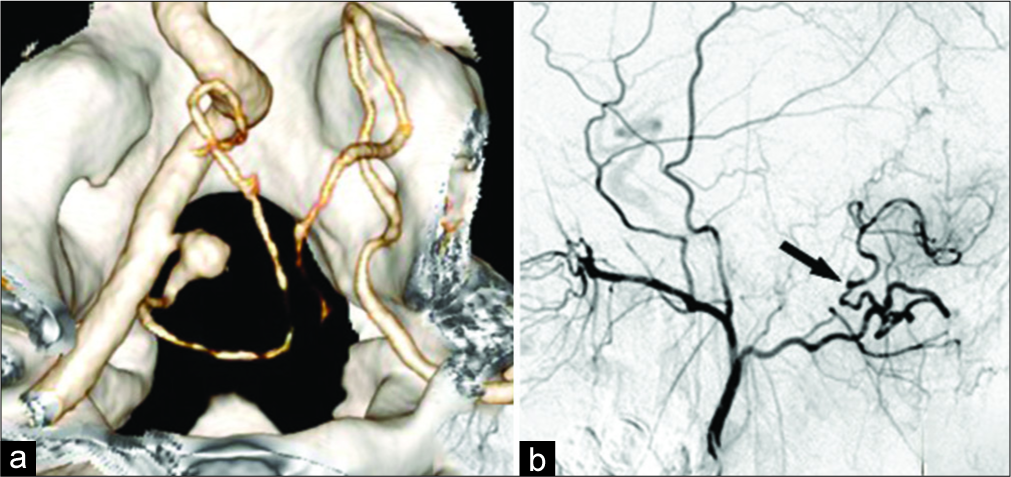
Figure 6:
Anastomosis between the occipital artery and the left PICA in a 20-year-old patient with PICAprox fusiform aneurysm. a. CT-angiography (3D reconstruction): fusiform aneurysm of the left PICAprox; b. Angiography of the left external carotid artery after trapping of the left PICAprox aneurysm and anastomosis creation (arrow) between the left occipital artery and PICA.
Deconstruction of the artery without anastomosis was possible in the VAdist fusiform aneurysms. The occlusion of this segment through proximal clipping was performed in two patients and trapping in one patient. The requirements for the VAdist occlusion with the aneurysm included PICA sparing (the clip was applied distal to the origin of the artery) and the presence of a second VA with comparable diameter to the main artery. Our attention settled on the fact that in fusiform aneurysms in the VA segment from the PICA origin to the VA confluence there were no large perforating arteries to the medulla oblongata. CALF waiving was due to the close association of this VA segment with the caudal cranial nerve group, the medulla oblongata, and the narrow surgical corridor [
Figure 7:
Trapping of the fusiform aneurysm of the right VAdist in female patient M., 43 years old. Vertebral angiography before surgery (a): the fusiform aneurysm of the right VAdist; CT angiography after surgery (b, c): 2 clips in the projection of the cut segment of the right VA and patent right PICA (arrow).
Intraoperative complications
Intraoperative aneurysm rupture occurred in 5 (6.3%) cases [
Two patients with PICAprox aneurysms and one patient with PICAdist aneurysms had no neurological complications after surgery.
One patient had moderate dysfunction of IX and X cranial nerve after clipping the PICAprox aneurysm.
In one female patient, the surgery was complicated by major intraoperative bleeding requiring clipping of the aneurysm with the right PICA origin that resulted in the postoperative ischemia in this region. Due to severe bulbar impairment, tracheostomy was performed. The patient was discharged from the hospital 90 days after surgery Glasgow outcome scale (GOS 3).
Intraoperative PICA thrombosis was reported in two (2.5%) patients [
In one patient with PICAprox aneurysm, a right PICA thrombosis in the area of its origin occurred after repeated clip reposition. An attempt of PICA reimplantation into the proximal VA segments was made; however, this anastomosis did not work. After surgery, the patient had right XII cranial nerve paresis. Postoperative computed tomography (CT) revealed no areas of brain ischemia. The patient was discharged on day 7 after surgery in good condition (GOS 4).
Another patient with a partially thrombosed fusiform aneurysm of the tonsillomedullary (p3) segment of the left PICA developed PICA thrombosis after a CALF attempt. Intraoperative attempts to restore blood flow in the distal PICA were unsuccessful. After surgery, the patient developed massive ischemia in the left hemisphere and the vermis with severe edema in the posterior cranial fossa. Two days after the initial operation, decompressive craniectomy of the posterior cranial fossa was performed. The patient had prolonged sedation with artificial ventilation through a tracheostomy tube. He was discharged on the 47th day after the surgery (GOS 3). At the time of discharge, the patient was conscious, had no movement disorders in the extremities; however, moderate bulbar disorders and severe cerebellar symptoms persisted.
Air embolism during craniotomy was reported in two (3.8%) of 52 patients operated in the sitting position. After hemostasis with occlusion of large veins in both cases, the operation was continued. In the postoperative period, one patient developed bilateral pneumonia in combination with moderate dysfunction of IX and X cranial nerves that required the placement of a tracheostomy tube.
Postoperative complications
Fifty-seven (71.3%) patients were discharged without worsening of the clinical signs after surgery [
Among patients with postoperative neurological disorders, the most common sign was palsy of IX and X cranial nerve (dysphonia, dysphagia, etc.) identified in 14 (17.5%) patients. In 3 cases, these were severe and required the placement of a tracheostomy tube during the recovery period.
In 11 patients, dysfunction of IX and X cranial nerves was observed with PICAprox aneurysms [
In three patients with signs of IX and X cranial nerve palsy, XII cranial nerve was also dysfunctional with tongue deviation and dysarthria.
In 3 cases of PICAprox aneurysms, an isolated palsy of the ipsilateral XII cranial nerve was reported.
Postoperative ischemic complications were observed in six patients. In three patients, aneurysm clipping was complicated by the PICA thrombosis and ischemic lesions in the cerebellar hemisphere.
In three patients, MRI showed small ischemic lesions in the medulla oblongata manifested with moderate hemiparesis in one patient and with hemihypesthesia in two others [
No postoperative hematomas and other hemorrhagic postoperative complications were reported.
Complicated postoperative wound healing occurred in three patients. In one case, external lumbar drainage was placed for 5 days due to the subcutaneous accumulation of cerebrospinal fluid in the postoperative wound area with subsequent recovery of this complication. Two patients had wound liquorrhea after surgery; revision surgery with the closure of the dura mater defects was performed. Subsequently, meningitis was diagnosed in one of these patients and etiotropic antibiotic therapy was administered with a good outcome.
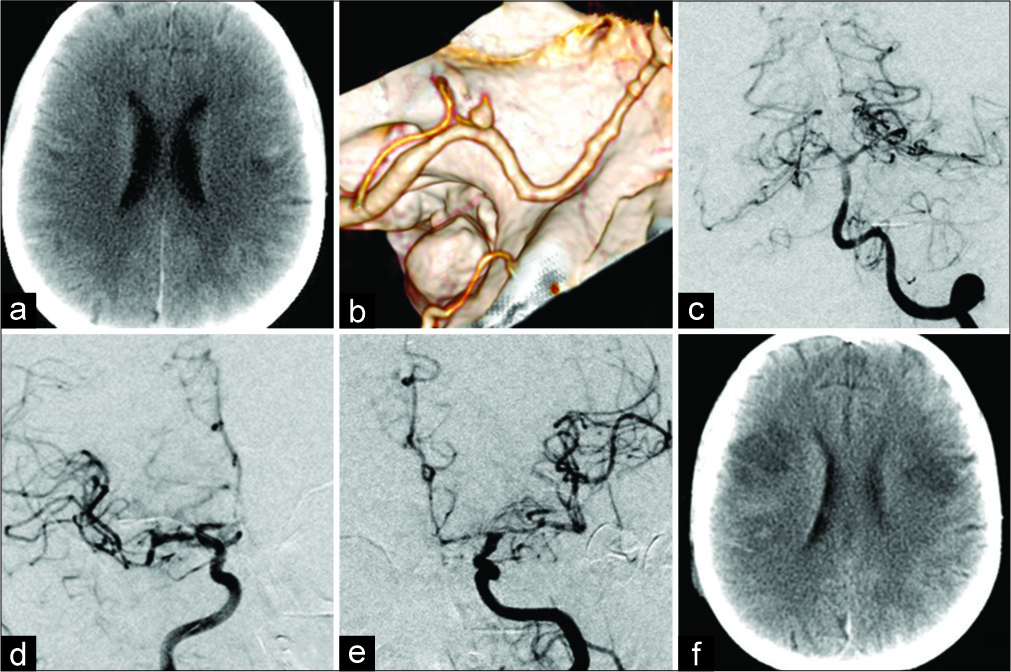
Postoperative complications associated with SAH were uncommon due to the relatively small number of patients in the acute period (12 patients). In one patient, operated 4 days after SAH, the focal hemispheric neurological signs developed 3 days after the surgery. Angiography revealed severe vasospasm in both MCA vascular regions that caused ischemic lesions in the cerebral hemispheres [
Figure 8:
Ischemic lesions in the cerebral hemispheres due to vasospasm after SAH from the PICAprox aneurysm in the female patient P, 60 years old. Brain CT on the day of surgery, 4 days after SAH (a); CT angiography before surgery (b): a small PICAprox aneurysm on the left; Cerebral angiography on day 3 after aneurysm clipping (c, d, e): the aneurysm is occluded, severe vasospasm in both carotid vascular regions; Brain CT on day 5 after surgery (f): ischemic lesions in both cerebral hemispheres.
Posthemorrhagic hydrocephalus was reported in 14 (18.7%) of 75 patients with SAH. In six patients, a ventriculoperitoneal shunt was implanted during the hospitalization.
Completeness of aneurysm occlusion
In the postoperative period, follow-up angiography was performed in 46 (57.5%) patients (digital subtraction angiography — 19, and CT-angiography — 27 patients). Complete aneurysm occlusion was confirmed in 43 (93.5%) cases, neck enhancement was revealed in 2 cases (4.3%), and enhancement of the neck and fundus – in one (2.2%) patient.
Patients with a partial enhancement of the aneurysm neck were closely monitored. Endovascular aneurysm coiling was performed in one patient with an enhancement of the aneurysm fundus.
In the other 34 patients without angiography, the complete aneurysm repair was verified by the ICG video angiography followed by opening the aneurysm sac.
Thus, the complete occlusion of PICA and VA aneurysms during microsurgery was achieved in 77 (96.3%) cases.
DISCUSSION
There are several points of view regarding surgical approaches to VA and PICA aneurysms.
Some authors claim that large basal approaches with partial resection of the occipital condyles provide better aneurysm exposure and reduced risk of postoperative dysfunction of the caudal cranial nerves (DCCN).[
In contrast, transcondylar approaches increase the total duration of the operation and increase the risk of poor wound healing, as well as craniocervical instability and neck pain.[
We agree with those authors who believe that the resection of condyles is unnecessary in the vast majority of cases.[
Indeed, the major challenge in the surgery of PICA and VA aneurysms involve neurovascular structures that obscure the neck of the aneurysm rather than bony prominences.[
We believe that the need for routine resection of the posterior arch of the atlas to approach the aneurysms of VA and PICA proposed by several authors[
Our recent experience has shown that retrosigmoid craniotomy is an adequate approach to the small PICAprox and VAdist aneurysms that are consistent with other authors.[
Actually, even significant bleeding from the VA or PICA aneurysms rare causes microsurgical difficulties. Al-khayat et al.[
One of six patients who underwent surgery within 14 days from the SAH event developed ischemic lesions in the cerebral hemispheres due to vasospasm. Estimated risk of angiospastic supratentorial cerebral ischemia in subtentorial SAH originating from the VA or PICA is 1.9–7%.[
Postoperative mortality in the surgical series of patients with VA and PICA aneurysms is 1.8–3.7%.[
Ischemic lesions in the cerebellum and brainstem in patients with PICA and VA aneurysms are primarily associated with surgical issues. In one study, postoperative ischemic cerebellar complications were diagnosed in 19% of patients.[
Unfortunately, there are no accurate predictive criteria of the risk for PICA occlusion yet.
We take guidance from the test with temporary PICA clipping and its retrograde enhancement in the ICG video angiography. However, its evaluation is simple only in extreme cases: the rapid intensive retrograde PICA enhancement along with other intact arteries or the complete absence of the enhancement. Currently, prolonged, mild, or moderate retrograde PICA enhancement may not predict the risk of ischemia. Therefore, we believe that in undetermined cases, revascularization of the target artery may be useful.
Seoane et al.[
Ipsilateral postoperative DCCN remains the main concern of microsurgery for VA and PICA aneurysms. According to the published data, the frequency of these complications after clipping of the aneurysm of VA and PICA varies from 7.4% to 29%.[
There are encouraging data that the rate of DCCN recovery within 6 months is over 76%.[
To reduce the risk of DCCN, sharp vasoneural dissection is recommended to prevent the tension of the IX, X, and XII cranial nerve, shorten the time for preventive clipping (up to 6 min) and avoid temporary VA trapping, if possible.[
The risk of DCCN increases with intraoperative ruptures.
The control of intraoperative bleeding in VA aneurysms includes the approachability of the proximal, and, in some cases, distal VA. The distal temporary clipping, especially in the segment between PICA and VA confluence, often requires medial traction of the medulla oblongata and carries the risk of damage to the caudal cranial nerve group.
Our study has shown that microsurgery provides an opportunity to achieve a high rate (95.5%) of the complete occlusion of VA and PICA aneurysms. According to the published data, the microsurgical technique has a high rate of complete aneurysm repair: 90–97.1%,[
In clinical series, including both microsurgical and endovascular methods, comparable clinical outcomes for aneurysms of VA and PICA were observed.[
However, comparison of our data with clinical and angiographic outcomes of endovascular treatment in the VA and PICA aneurysms is inappropriate as we initially included in the study patients selected for microsurgical treatment according to the above criteria rather than consecutive patients. Therefore, it seems promising to use a combined technique in multiple aneurysms, as well as in giant VA aneurysms where the endovascular deconstruction of the parent artery is possible in combination with microsurgical revascularization and elimination of the mass effect.
The limitations of our study included: a retrospective design, the fact that in most patients, SAH was treated on a delayed basis, and the lack of follow-up data beyond discharge.
CONCLUSION
Microsurgical treatment is an effective method for VA and PICA aneurysms.
The majority of VA and PICA aneurysms do not require complex basal approach with resection of the condyles and cervical vertebrae.
A thorough preoperative planning using advanced neuroimaging methods, reconstructive clipping techniques, and anastomoses creation, as well as patient selection based on the established algorithms and consultations with endovascular surgeons, may reduce the number of complications and increase the rate of complete microsurgical repair in VA and PICA aneurysms.
References
1. Abla AA, McDougall CM, Breshears JD, Lawton MT. Intracranial-to-intracranial bypass for posterior inferior cerebellar artery aneurysms: Options, technical challenges, and results in 35 patients. J Neurosurg. 2016. 124: 1275-86
2. Al-khayat H, Beshay J, Manner D, White J. Vertebral artery-posteroinferior cerebellar artery aneurysms: Clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005. 56: 2-10
3. Aronov M, Mokin M, Zelenkov A, Popugaev K, Tsarikaev A, Reutov A. Endovascular coiling of ruptured very small dissecting fusiform aneurysm of posterior inferior cerebellar artery with parent artery preservation by microcatheter auto-assistance. World Neurosurg. 2019. 121: 152-5
4. Bertalanffy H, Sure U, Petermeyer M, Becker R, Gilsbach JM. Management of aneurysms of the vertebral artery-posterior inferior cerebellar artery complex. Neurol Med Chir (Tokyo). 1998. 38: 93-103
5. Bohnstedt BN, Ziemba-Davis M, Edwards G, Brom J, Payner TD, Leipzig TJ. Treatment and outcomes among 102 posterior inferior cerebellar artery aneurysms: A comparison of endovascular and microsurgical clip ligation. World Neurosurg. 2015. 83: 784-93
6. Bradac GB, Bergui M. Endovascular treatment of the posterior inferior cerebellar artery aneurysms. Neuroradiology. 2004. 46: 1006-11
7. Chalouhi N, Jabbour P, Starke RM, Tjoumakaris SI, Gonzalez LF, Witte S. Endovascular treatment of proximal and distal posterior inferior cerebellar artery aneurysms. J Neurosurg. 2013. 118: 991-9
8. D’Ambrosio AL, Kreiter KT, Bush CA, Sciacca RR, Mayer SA, Solomon RA. Far lateral suboccipital approach for the treatment of proximal posteroinferior cerebellar artery aneurysms: Surgical results and long-term outcome. Neurosurgery. 2004. 55: 39-50
9. Eliava SS, Yakovlev SB, Belousova OB, Pilipenko YV, Kheyreddin AS, Shekhtman OD. The principles for choosing a surgical technique for patients with acute cerebral aneurysm rupture. Zh Vopr Neirokhir Im N N Burdenko. 2016. 80: 15-21
10. Eliava SS, Yakovlev SB, Shekhtman OD, Pilipenko YV, Kheyreddin AS, Konovalov AN. Principles of surgical treatment for patients with asymptomatic aneurysms and cerebral aneurysms in the cold period after spontaneous intracranial hemorrhages. Zh Vopr Neirokhir Im N N Burdenko. 2018. 82: 8-14
11. Lehto H, Harati A, Niemelä M, Dashti R, Laakso A, Elsharkawy A. Distal posterior inferior cerebellar artery aneurysms: Clinical features and outcome of 80 patients. World Neurosurg. 2014. 82: 702-13
12. Lemole GM, Henn J, Javedan S, Deshmukh V, Spetzler RF. Cerebral revascularization performed using posterior inferior cerebellar artery-posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg. 2002. 97: 219-23
13. Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL. Distal posterior inferior cerebellar artery aneurysms: Clinical features and management. J Neurosurg. 2002. 97: 756-66
14. Lister JR, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982. 10: 170-99
15. Mericle RA, Reig AS, Burry MV, Eskioglu E, Firment CS, Santra S. Endovascular surgery for proximal posterior inferior cerebellar artery aneurysms: An analysis of glasgow outcome score by hunt-hess grades. Neurosurgery. 2006. 58: 619-25
16. Mikeladze KG, Okishev DN, Belousova OB, Konovalov AN, Pilipenko YV, Kheireddin AS. Intra-arterial administration of verapamil for prevention and treatment of cerebral angiospasm after SAH due to cerebral aneurysm rupture. Zh Vopr Neirokhir Im N N Burdenko. 2018. 82: 23-31
17. Mintelis A, Sameshima T, Bulsara KR, Gray L, Friedman AH, Fukushima T. Jugular tubercle: Morphometric analysis and surgical significance. J Neurosurg. 2006. 105: 753-7
18. Nussbaum ES, Mendez A, Camarata P, Sebring L. Surgical management of fusiform aneurysms of the peripheral posteroinferior cerebellar artery. Neurosurgery. 2003. 53: 831-4
19. Orakcioglu B, Schuknecht B, Otani N, Khan N, Imhof HG, Yonekawa Y. Distal posterior inferior cerebellar artery aneurysms: Clinical characteristics and surgical management. Acta Neurochir (Wien). 2005. 147: 1131-9
20. Peerless SJ, Drake CG, Youmans JR.editors. Management of aneurysms of the posterior circulation. Neurological Surgery. Philadelphia, PA: W.B. Saunders Co; 1990. 3:
21. Petr O, Sejkorová A, Bradáč O, Brinjikji W, Lanzino G. Safety and efficacy of treatment strategies for posterior inferior cerebellar artery aneurysms: A systematic review and meta-analysis. Acta Neurochir (Wien). 2016. 158: 2415-28
22. Salcman M, Rigamonti D, Numaguchi Y, Sadato N. Aneurysms of the posterior inferior cerebellar artery-vertebral artery complex: Variations on a theme. Neurosurgery. 1990. 27: 12-20
23. Sejkorová A, Petr O, Mulino M, Cihlář J, Hejčl A, Thomé C. Management of posterior inferior cerebellar artery aneurysms: What factors play the most important role in outcome?. Acta Neurochir (Wien). 2017. 159: 549-58
24. Seoane P, Kalb S, Clark JC, Rivas JC, Xu DS, Mendes GAC. Far-lateral approach without drilling the occipital condyle for vertebral artery-posterior inferior cerebellar artery aneurysms. Neurosurgery. 2017. 81: 268-74
25. Song J, Park JE, Chung J, Lim YC, Shin YS. Treatment strategies of ruptured posterior inferior cerebellar artery aneurysm according to its segment. Surg Neurol Int. 2017. 8: 155-
26. Srinivasan VM, Ghali MG, Reznik OE, Cherian J, Mokin M, Dumont TM. Flow diversion for the treatment of posterior inferior cerebellar artery aneurysms: A novel classification and strategies. J Neurointerv Surg. 2018. 10: 663-8
27. Tjahjadi M, Rezai Jahromi B, Serrone J, Nurminen V, Choque-Velasquez J, Kivisaari R. Simple lateral suboccipital approach and modification for vertebral artery aneurysms: A Study of 52 cases over 10 years. World Neurosurg. 2017. 108: 336-46
28. Viswanathan GC, Menon G, Nair S, Abraham M. Posterior inferior cerebellar artery aneurysms: Operative strategies based on a surgical series of 27 patients. Turk Neurosurg. 2014. 24: 30-7