- Department of Radiology, The Jikei University Hospital, Minato City, Tokyo, Japan
- Department of Neurosurgery, The Jikei University School of Medicine, Minato City, Tokyo, Japan
- Siemens Healthcare K.K., Shinagawa City, Tokyo, Japan.
Correspondence Address:
Toshihiro Ishibashi, MD PhD Department of Neurosurgery, The Jikei University School of Medicine, 3-25-8 Nishi- Shinbashi, Minato-Ku 105- 8461, Tokyo, Japan.
DOI:10.25259/SNI_675_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yukiko Abe1, Toshihiro Ishibashi2, Katharina Otani3, Issei Kan2, Yuichi Murayama2. Virtual coil images can optimize the visualization of the neckline of intracranial aneurysms during coil embolization: A technical note. 29-Sep-2023;14:349
How to cite this URL: Yukiko Abe1, Toshihiro Ishibashi2, Katharina Otani3, Issei Kan2, Yuichi Murayama2. Virtual coil images can optimize the visualization of the neckline of intracranial aneurysms during coil embolization: A technical note. 29-Sep-2023;14:349. Available from: https://surgicalneurologyint.com/surgicalint-articles/12571/
Abstract
Background: During coil embolization of intracranial aneurysms, the aneurysmal neck needs to be evaluated because coil protrusion into the parent artery may lead to ischemic complications. However, the neck cannot always be clearly visualized due to the limitation of the angiography system and due to the structure of the aneurysm. As a visual aid, we propose a color-coded fusion imaging method that generates “virtual coil” images using preoperative three-dimensional digital subtraction angiography (3D-DSA) images.
Case Description: Coil embolization for intracranial aneurysms was performed using the working angles determined from the preoperative 3D-DSA. The aneurysms were located at the middle cerebral artery, anterior communicating artery (A-com), and posterior communicating artery (P-com). The A-com and P-com aneurysms were recurrent. During the later phase of the procedure, physicians could not judge whether coils protruded into the parent artery on two-dimensional digital subtraction angiography (2D-DSA) images because an optimal working angle could not be realized. Virtual coil images were displayed on the angiography system’s monitor to show the expected completed embolization, which could be compared to the current 2D-DSA images as a visual aid.
Conclusion: Virtual coil images can provide visual aid to the treating physician during aneurysm coil embolization, which is useful when an accurate working angle cannot be reached.
Keywords: Three-dimensional digital subtraction angiography, Coil protrusion, Fusion, Intracranial aneurysm, Virtual coil embolization, Image fusion, Simulation
INTRODUCTION
Progress in diagnostic imaging technology has contributed to improved safety of intracranial endovascular treatments. Stent-assisted coil embolization of cerebral aneurysms heavily relies on precise imaging, and an important factor for evaluating the success of the treatment is whether coils protrude into the neck of the aneurysm. This is because coil protrusion into the parent artery is considered being one of the risk factors for ischemic complications.[
The workflow of coil embolization often starts with the acquisition of a preoperative 3D-DSA and planning the two optimal working angles for the 2D-DSA views. One view will aim at having a cross-section of the aneurysmal neck along the parent artery, and the other view will aim at a down the barrel view.[
To overcome these limitations, we propose a color-coded image post processing method for 3D-DSA images, “virtual coil.” The virtual coil would allow us to understand the area to be embolized at one glance, even in cases when an accurate working angle cannot be reached by the angiography system. Virtual coil also would allow us to judge the presence or absence of coil protrusion more accurately when on 2D-DSA the coil seems to protrude into the parent artery.
VIRTUAL COIL IMAGING
This study was approved by the Institutional Review Board (IRB) (27-236[8121]). In this case series, all image data were acquired by a biplane angiography system (Artis Q, Siemens Healthcare GmbH, Forchheim, Germany) and post processed on the workstation (syngo XWP, Siemens Healthcare GmbH) of the system. The 3D-DSA preoperative image data were used to reconstruct two subtraction images using the following parameters: HU kernel type, normal image characteristics, and slice matrix of 512 × 512, resulting in a voxel size of approximately 0.15 mm in each dimension. In the first image, the aneurysm was cut out manually and colored as a solid image, and the second image was displayed as a translucent image including the aneurysm and all vessel structures by modifying the trapezoid transfer function in the volume rendering technique. Both images were fused on the workstation resulting in “virtual coil” images of a translucent parent artery with a solid-colored aneurysm overlay.
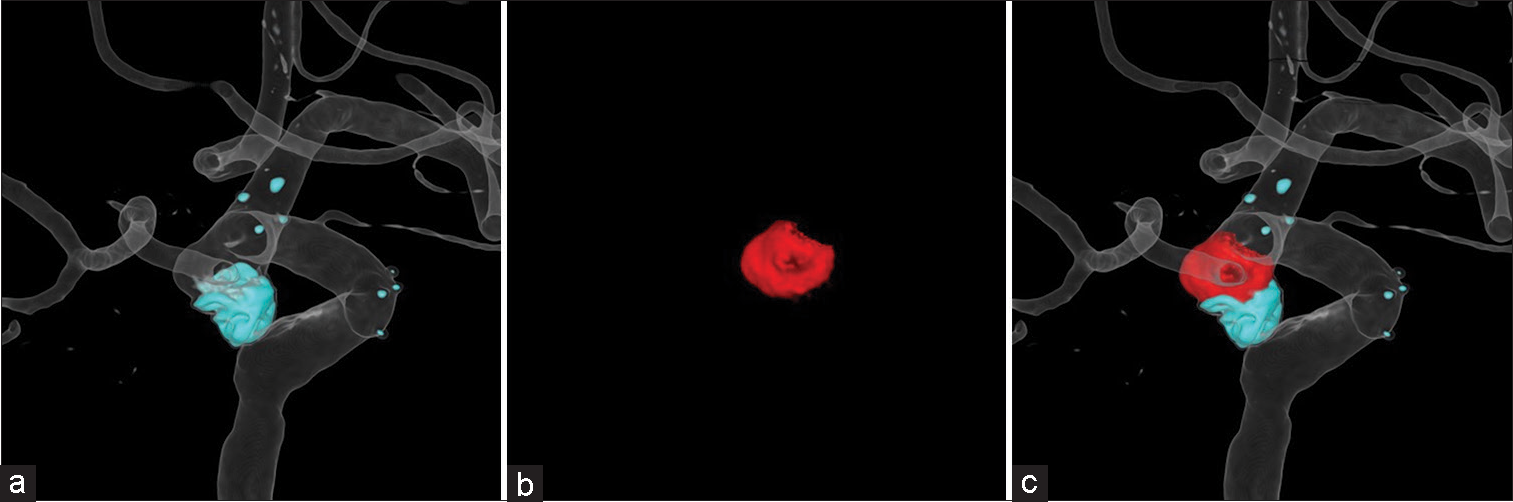
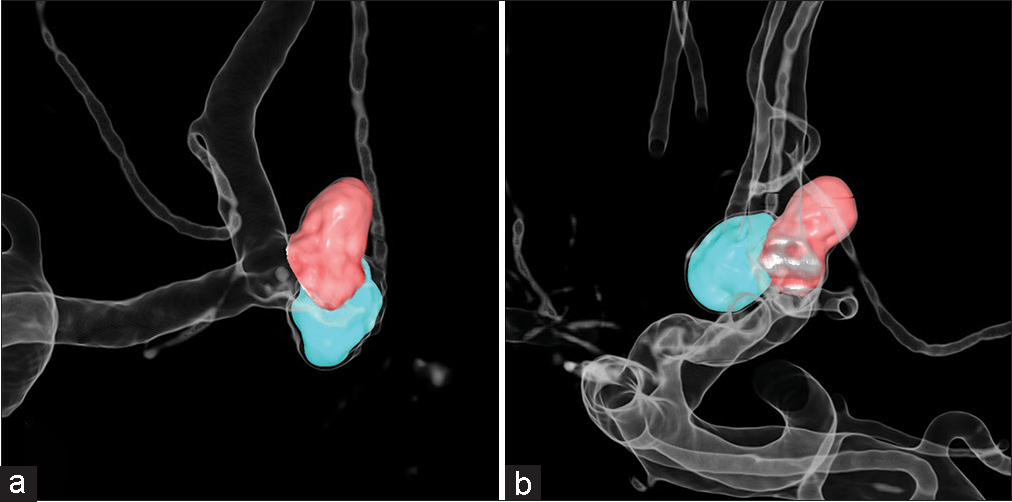
In re-embolization cases, virtual coil images were created using contrast-enhanced images and subtraction images. The contrast-enhanced images were used to show the parent artery and coil mass of the first embolization and the subtraction images were used to show the area to be reembolized. The parent artery was displayed as a translucent image, and coil mass and new aneurysm were displayed as solid-colored images [
Figure 1:
Generation of virtual coil images: (a) Images of the coil mass from the first embolization and parent artery after adjusting the trapezoid transfer function of the contrast-enhanced images in the volume rendering technique. (b) The area to be re-embolized was manually cut from the subtraction images (red). (c) Virtual coil images after fusion to differentiate coils (blue) and area to be re-embolized (red).
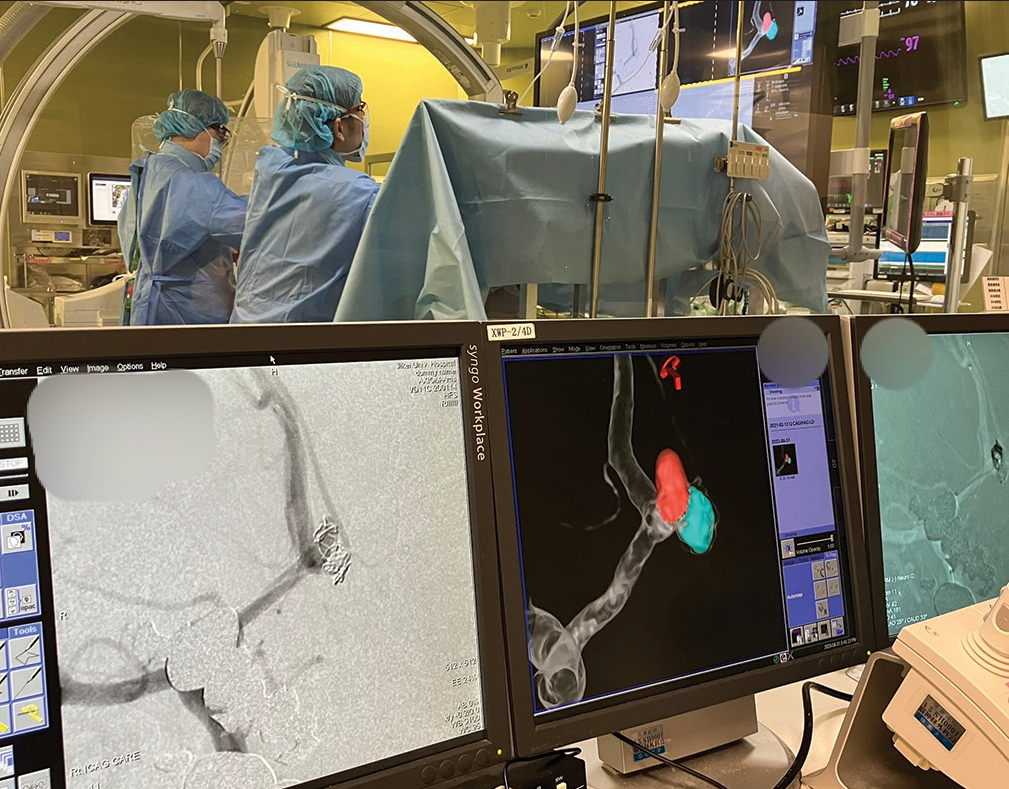
In this case series, a virtual coil image was used for the determination of the working angle and the comparison with the 2D-DSA images during each coil embolization procedure.
RESULTS
Case 1: MCA aneurysm
A 50-year-old woman was diagnosed with an incidental aneurysm located at MCA via magnetic resonance imaging (MRI). The aneurysm with a maximum size of 5.2 mm was located at the junction of M1-M2, and diagnostic angiography examination revealed a complex shape of the aneurysm. The parent artery was found to cover the aneurysm, and complete embolization was expected to be difficult.
Stent-assisted coil embolization was performed under general anesthesia. The working angles predicted from the diagnostic angiography were around the left anterior oblique (LAO) 60° and the right anterior oblique (RAO) 50°. For the procedure, the patient’s head was tilted to the right side about 30° before the embolization to optimize the position of the two planes of the angiography system. The working angles decided based on the 3D-DSA image acquired at the beginning of the procedure could not separate the aneurysm and parent artery due to the complex shape of the aneurysm [
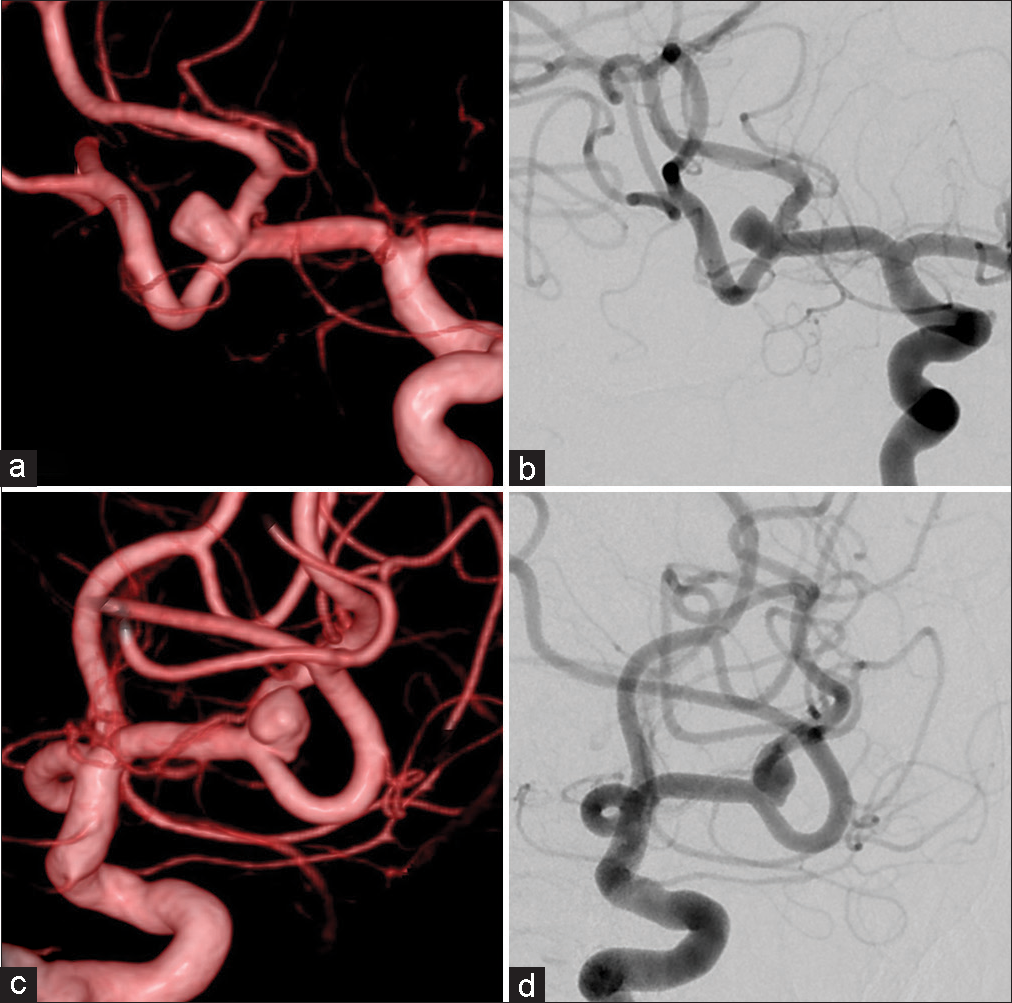
To facilitate visualization of the aneurysm for embolization, virtual coil images were generated from the 3D-DSA image [
Figure 3:
Case 1: (a and b) Virtual coil images generated from the preoperative three-dimensional digital subtraction angiography (DSA) images with the aneurysm area to be coiled (red). (c and d) Two-dimensional DSA after the embolization shows the coil masses as suggested by the virtual coil images.
Case 2: A-com aneurysm
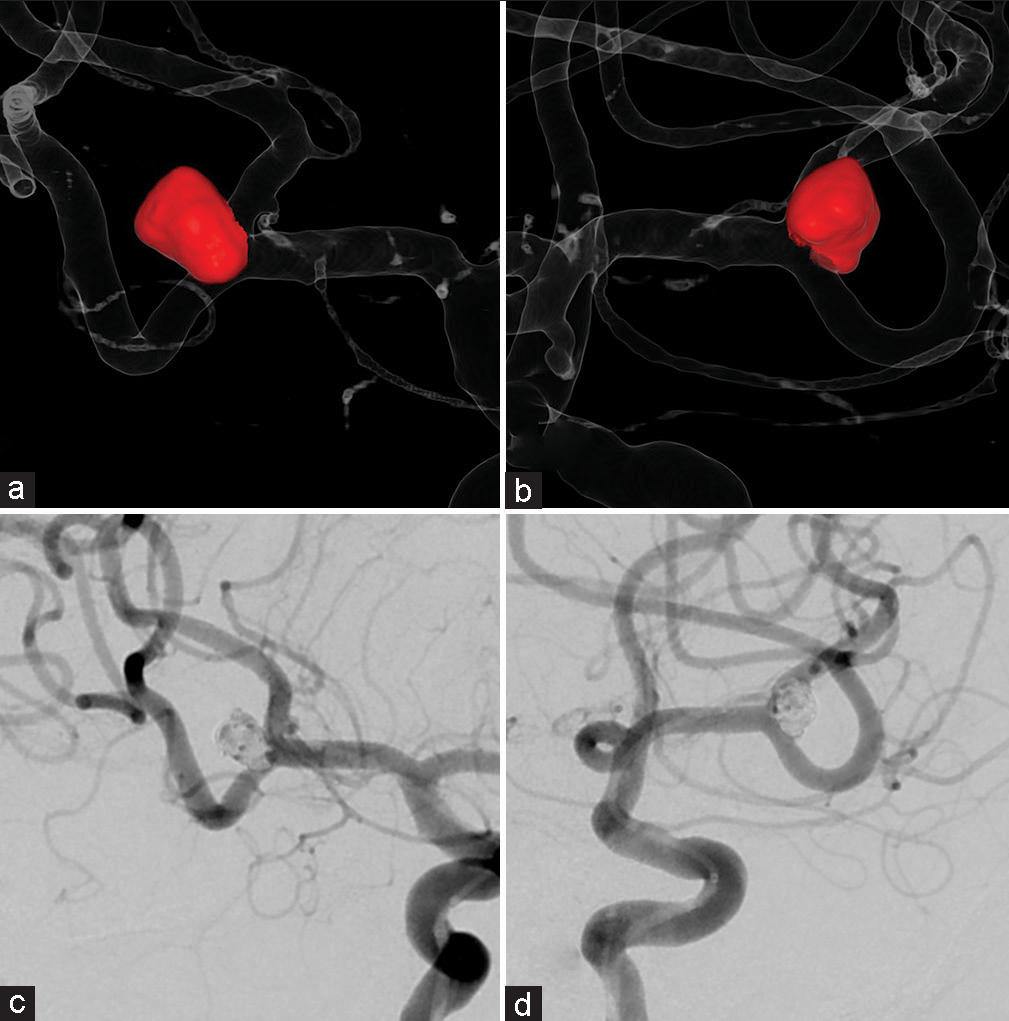
A 59-year-old man had been diagnosed with an incidental aneurysm located at the A-com which was treated with coil embolization. Follow-up by non-contrast MRI was continued for several years. Recanalization increased gradually and retreatment with stent assist coil embolization was scheduled. Recanalization occurred at a slightly different location than that of the first aneurysm. A de novo-like bump was observed in front of the coil mass [
Coil embolization and stent placement were performed using the two working angles of neck view and barrel view. Virtual coil images were displayed on the monitor during the procedure and compared to the 2D-DSA images [
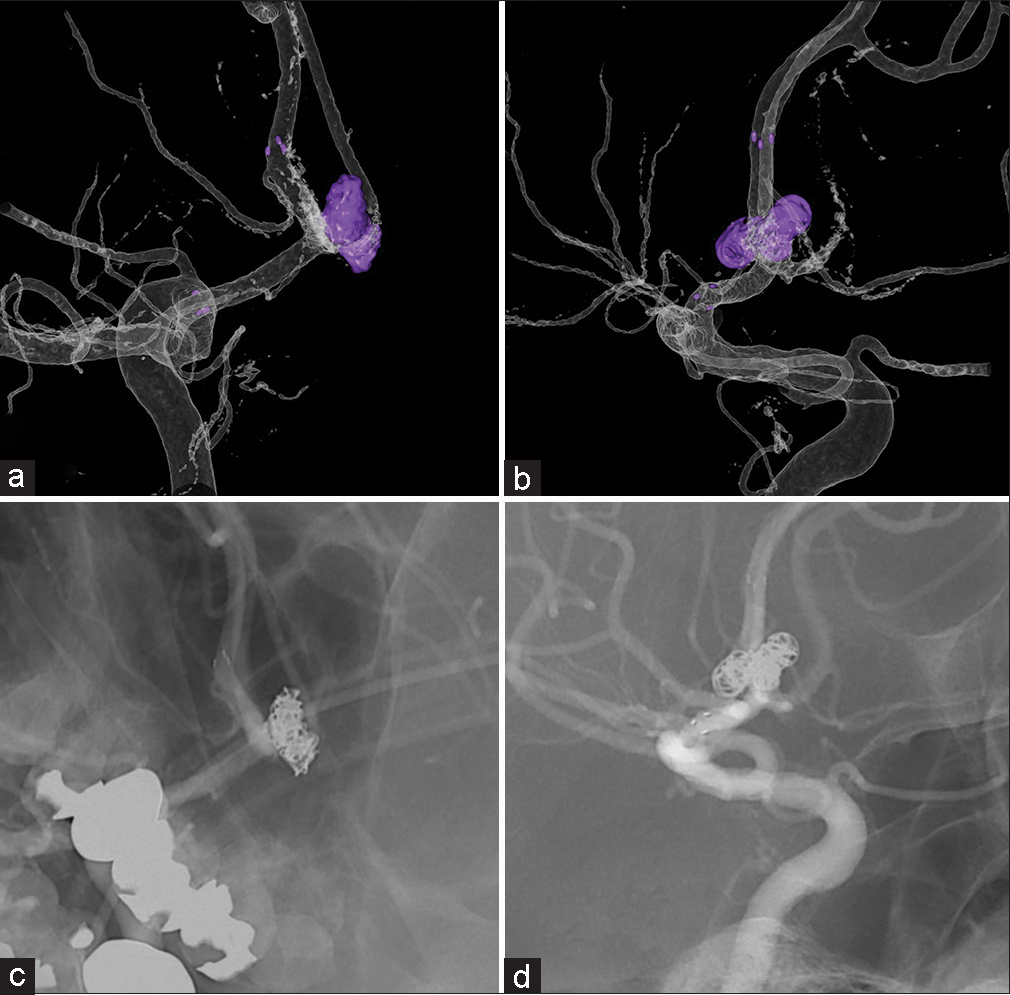
Figure 7:
Case 2: (a and b) Three-dimensional digital subtraction angiography images of the total coil mass (purple) after the retreatment. (c and d) Corresponding two-dimensional digital subtraction angiography images. The shape of the new coil mass matches the mass shown on the virtual coil images in Figure 5.
Case 3: Posterior communicating artery (P-com) aneurysm
A 77-year-old woman was diagnosed with an incidental left P-com aneurysm with a maximum size of 6 mm during a brain checkup. The aneurysm was treated by stent-assisted coil embolization, but a neck remnant remained, and observation by annual checkups was decided. After 8 years, the patient experienced oculomotor nerve paralysis. A diagnostic angiography revealed cross flow of the P-com and retreatment by coil embolization including the P-com was scheduled.
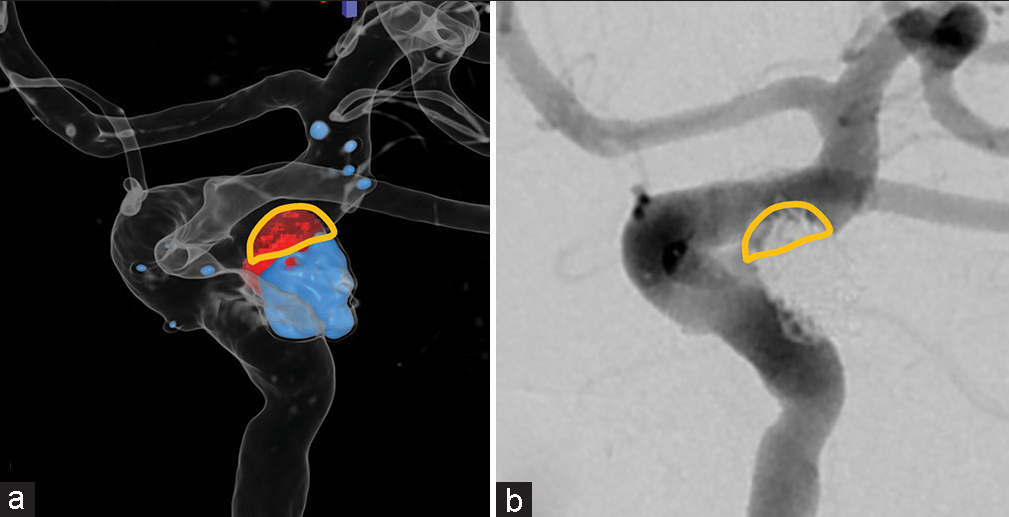
The procedure started with the acquisition of a cone-beam CT image to assess the situation of the placed stent, and acquisition of a 3D-DSA image to create a virtual coil image. The retreatment was planned as the embolization of the P-com together with the aneurysm. Two working angles were determined using the 3D-DSA image, but the angles that would clearly separate the aneurysm and parent artery could not be achieved during the procedure due to the limitation of the angiography system [
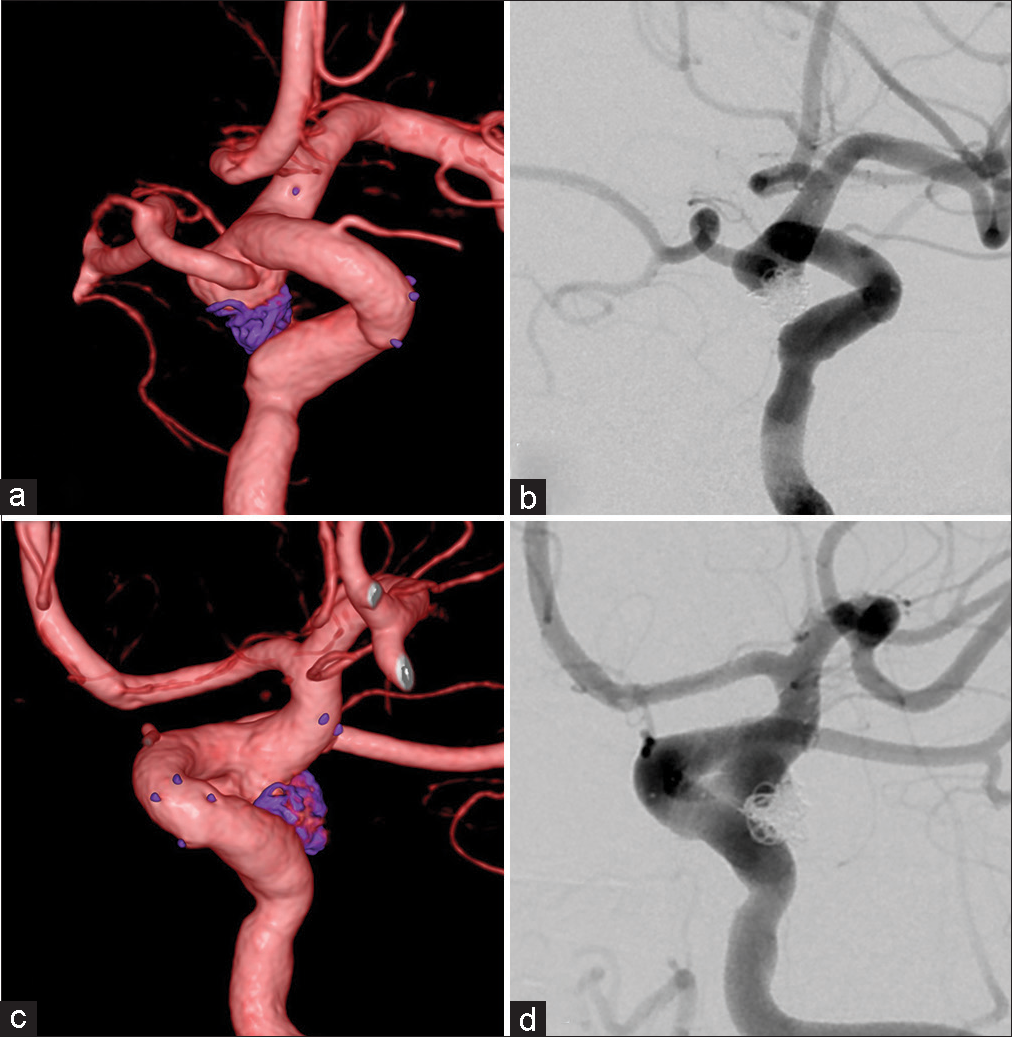
Figure 8:
Case 3: (a and b) Barrel view (three-dimensional digital subtraction angiography [3D-DSA] and two-dimensional digital subtraction angiography [2D-DSA]) achieved by the angiography system. (c and d) View aiming at separating the aneurysm and parent artery (3D-DSA and 2D-DSA) could not be achieved.
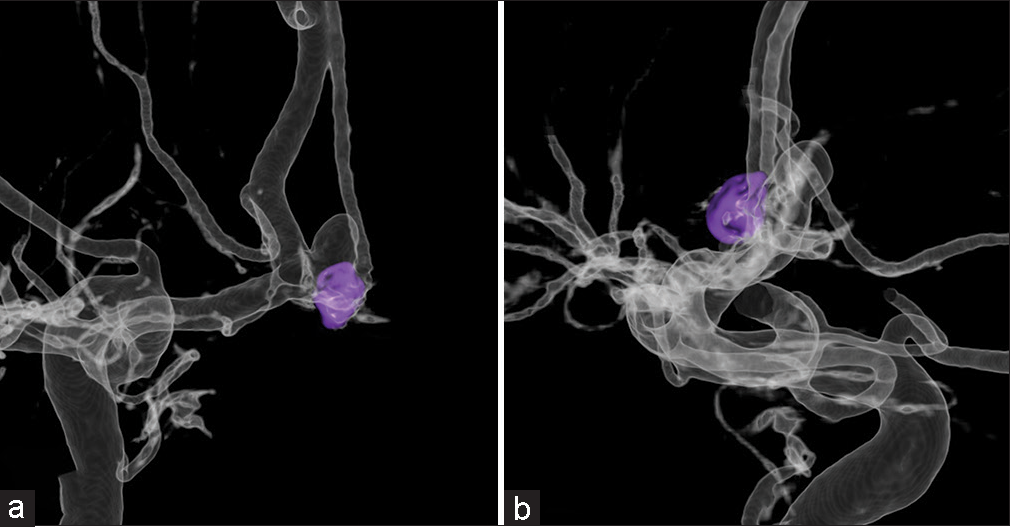
Figure 9:
Case 3: (a) Preoperative virtual coil image with coil mass from the first coil embolization (blue), coil mass to be deployed during retreatment (red). (b) Postoperative 2-dimensional digital subtraction angiography of the same view as the virtual coil images. Yellow outline shows the overlap area of the aneurysm and parent artery.
DISCUSSION
Coil protrusion during coil embolization for cerebral aneurysm is not rare, with incidence estimates ranging from 5.2% to 18.9%.[
Virtual coil images could be generated in a few minutes during the procedure with commercially available software installed on the workstation of the angiography system and may facilitate the determination of working angles of the procedure. The images display the aneurysm and parent vessel in different colors to show the area where coils will be deployed, which helps the treating physician understand the spatial relationship between the aneurysm, the existing coils if present, the area that needs to be embolized and the parent artery. Virtual coil images can be displayed side-by-side next to live and 2D-DSA images, allowing the treating physician to confirm coil protrusion in real-time in the operation room. Furthermore, it is useful as a tool to explain the area of the embolization to patients. In this case series, virtual coil images were useful for deciding working angles for treatments of medium-sized internal carotid artery aneurysms with a shared neck, A-com, and MCA aneurysms with bifurcation. Virtual coil images also suggested the final shape of the embolization.
Workstations equipped with an application that automatically separates the aneurysm and parent vessel and calculates the aneurysm volume have been reported to be useful in selecting the appropriate device size for web implantation.[
This method has some limitations. The separation of the aneurysm and parent vessel depends on the operator because the segmentation is done manually. Furthermore, the shape and angle of the vessels may change when the coils and microcatheters are deployed into the aneurysm because the virtual coil images were fused using preoperative 3D-DSA images. Virtual coil images thus become less precise as the procedure progresses. Furthermore, some shapes of aneurysms are less suitable for the generation of virtual coil images, such as aneurysms which have a complex aneurysmal neck and spindle-shaped aneurysms, because the aneurysmal neck cannot be defined accurately. Finally, the 3D-DSA image tends to overestimate the area filled with contrast medium due to volume averaging.[
Nevertheless, we believe that virtual coil image may help the treating physician, as it provides a visual aid for the treatment.
CONCLUSION
Virtual coil images can display the area of coil embolization when an accurate working angle cannot be reached because of limitations of the angiography system or because of the structural relationship of the aneurysm and surrounding vessels and provide visual aid to the treating physician during aneurysm coil embolization.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
The Jikei University received research funding from Siemens Healthcare K. K. and Stryker Japan K. K. for other unrelated research projects.
Conflicts of interest
Dr. Katharina Otani is an employee of Siemens Healthcare.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abdihalim M, Kim SH, Maud A, Suri MF, Tariq N, Qureshi AI. Short-and intermediate-term angiographic and clinical outcomes of patients with various grades of coil protrusions following embolization of intracranial aneurysms. Am J Neuroradiol. 2011. 32: 1392-8
2. Ansari S, Zevallos CB, Farooqui M, Dajles A, Schafer S, Quispe-Orozco D. Optimal woven endobridge (WEB) device size selection using automated volumetric software. Brain Sci. 2021. 11: 901
3. Brinjikji W, Cloft H, Lanzino G, Kallmes DF. Comparison of 2D digital subtraction angiography and 3D rotational angiography in the evaluation of dome-to-neck ratio. Am J Neuroradiol. 2009. 30: 831-4
4. Chan SH, Wong KS, Woo YM, Chan KY, Leung KM. Volume measurement of the intracranial aneurysm: A discussion and comparison of the alternatives to manual segmentation. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 358-63
5. Fiorella D, Albuquerque FC, Masaryk TJ, Rasmussen PA, McDougall CG. Balloon-in-stent technique for the constructive endovascular treatment of “ultra-wide necked” circumferential aneurysms. Neurosurgery. 2005. 57: 1218-26
6. Ishihara H, Ishihara S, Niimi J, Neki H, Kakehi Y, Uemiya N. Risk factors for coil protrusion into the parent artery and associated thrombo-embolic events following unruptured cerebral aneurysm embolization. Interv Neuroradiol. 2015. 21: 178-83
7. Lee D, Lee DH, Park JC, Shin JH, Song Y, Chung J. Timing of thrombosis in embolization of unruptured intracranial aneurysms: Tirofiban as rescue treatment. Clin Neuroradiol. 2021. 31: 125-33