- Department of Neurosurgery, Sapporo Medical University, Sapporo, Hokkaido, Japan,
- Department of Endovascular Neurosurgery, Saitama Medical University, International Medical Center, Hidaka, Saitama, Japan,
- Division of Radiology, Sapporo Medical University Hospital, Sapporo, Hokkaido, Japan,
- Division of Radiology, Sapporo Shiroishi Memorial Hospital, Sapporo, Hokkaido, Japan,
- Department of Neurosurgery, Sapporo Shiroishi Memorial Hospital, Sapporo, Hokkaido, Japan.
Correspondence Address:
Tomoyoshi Kuribara, Department of Neurosurgery, Sapporo Medical University, Sapporo, Hokkaido, Japan.
DOI:10.25259/SNI_439_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tomoyoshi Kuribara1, Takeshi Mikami1, Satoshi Iihoshi2, Toru Hirano3, Daisuke Sasamori4, Tadashi Nonaka5, Nobuhiro Mikuni1. Virtual test occlusion for assessing ischemic tolerance using computational fluid dynamics. 27-Jul-2021;12:378
How to cite this URL: Tomoyoshi Kuribara1, Takeshi Mikami1, Satoshi Iihoshi2, Toru Hirano3, Daisuke Sasamori4, Tadashi Nonaka5, Nobuhiro Mikuni1. Virtual test occlusion for assessing ischemic tolerance using computational fluid dynamics. 27-Jul-2021;12:378. Available from: https://surgicalneurologyint.com/surgicalint-articles/10992/
Abstract
Background: Ischemic tolerance has been evaluated by the balloon test occlusion (BTO) for cerebral aneurysms and tumors that might require parent artery occlusion during surgery. However, because of its invasiveness, a non-invasive evaluation method is needed. In this study, we assessed the possibility of virtual test occlusion using computational fluid dynamics (CFD) as a non-invasive alternative to BTO for evaluating ischemic tolerance.
Methods: Twenty-one patients who underwent BTO were included in the study. Virtual test occlusion was performed using CFD analysis, and the flow rate (FR) and wall shear stress (WSS) of the middle cerebral artery on the occlusion side were calculated. The correlations between these parameters and examination data including the parameters of computed tomography perfusion during BTO were assessed and the cutoff value of CFD parameters for detecting the good collateral group was calculated.
Results: The FR was strongly correlated with mean transit time (MTT) during BTO and moderately correlated with collateral flow grade based on angiographic appearance. The WSS was moderately correlated with collateral flow grade, mean stump pressure (MSP), and MTT. Furthermore, the FR and WSS were strongly correlated with the total FR and the diameters of the inlet vessels. The cutoff value of FR for detecting the good collateral group was 126.2 mL/min, while that of the WSS was 4.54 Pa.
Conclusion:
Keywords: Balloon test occlusion, Computational fluid dynamics, Computed tomography perfusion, Flow rate, Wall share stress
INTRODUCTION
Ischemic tolerance has been evaluated by balloon test occlusion (BTO) for large and giant cerebral aneurysms[
Image-based computational fluid dynamics (CFD) are able to extract patient-specific hemodynamic information based on the imaging data obtained through CT angiography (CTA), 3-dimensional (3D) DSA, and magnetic resonance angiography.[
MATERIALS AND METHODS
Patients
The study protocol was approved by the ethics committee of Sapporo Medical University Hospital. As this study had a retrospective design, patient consent was obtained with an opt-out policy using a website. From March 2015 to February 2020, consecutive patients who underwent BTO of the ICA at our hospital were enrolled in the study. The patients were diagnosed with cerebral aneurysms, head and neck tumors, and other conditions (intracranial infection) that might require parent artery occlusion during surgery. Patients under 10-years-old and those who required BTO of other arteries were excluded from the study. A total of 21 patients (seven males and 14 females) were examined. The median age (interquartile range) of the patients was 61.0 (50.0–69.5) years (range, 11–74 years). The diagnoses of the 21 patients included cerebral aneurysm in four; head and neck tumor in 15; and other in two.
BTO
All procedures were performed using single-plane DSA equipment (Infinix Celeve-i INFX-8000C, Canon Inc., Tokyo, Japan) as previously described.[
Stump pressure was measured using a 3.3-Fr MASAMUNE balloon catheter (Fuji Systems Corp., Tokyo, Japan) placed in the ICA on the lesion side. The ratio of the mean stump pressure (MSP) before and after the temporal occlusion adjusted by mean blood pressure taken from a cuff on the left upper arm was calculated as MSPBR ([MSP 10 min after occlusion/mean blood pressure 10 min after occlusion]/[MSP before occlusion/mean blood pressure before occlusion]) (BR; ratio before and after temporal occlusion).
The INVOS examination was performed during the temporary occlusion. The ratio of INVOS before versus after the temporal occlusion adjusted by INVOS on the contralateral side was calculated as INVOSBR = ([INVOS on the lesion side 10 min after the occlusion/INVOS on the contralateral side 10 min after the occlusion]/[INVOS on the lesion side before the occlusion/INVOS on the contralateral side before the occlusion]).
If the temporary occlusion was <10 min due to ischemic symptoms, MSP and INVOS values just before balloon deflation were used.
CT perfusion during BTO
CT perfusion was performed using interventional radiology CT equipment with 16 rows (Aquilion 16 LB; Canon Inc.). The CT perfusion scanning parameters were described previously.[
Virtual test occlusion based on CFD analysis
The CTA examination was performed before BTO using a multidetector-row CT scanner with 320 rows (Aquilion ONE; Canon Inc.). The 3D-CTA scanning parameters were as previously described.[
Figure 1:
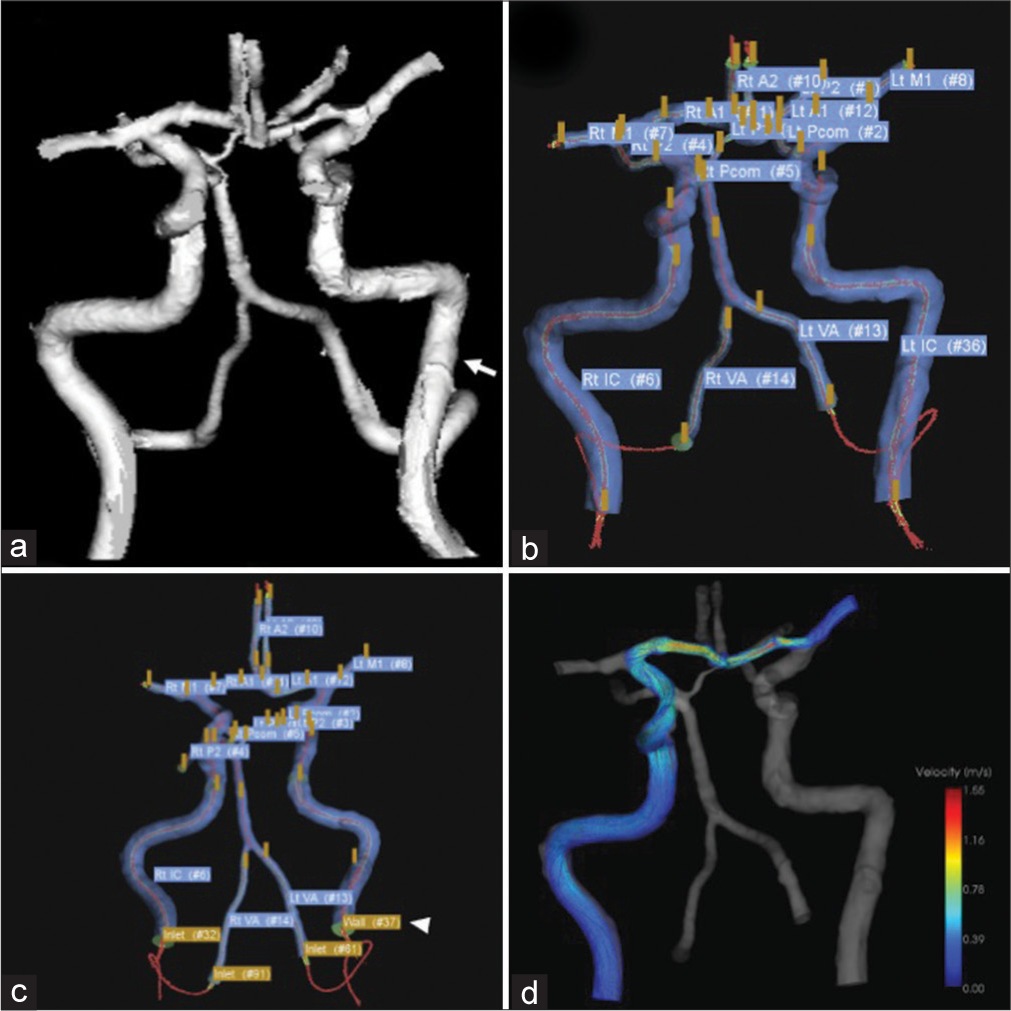
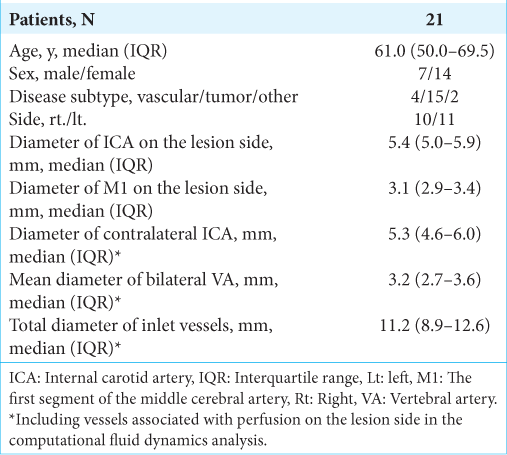
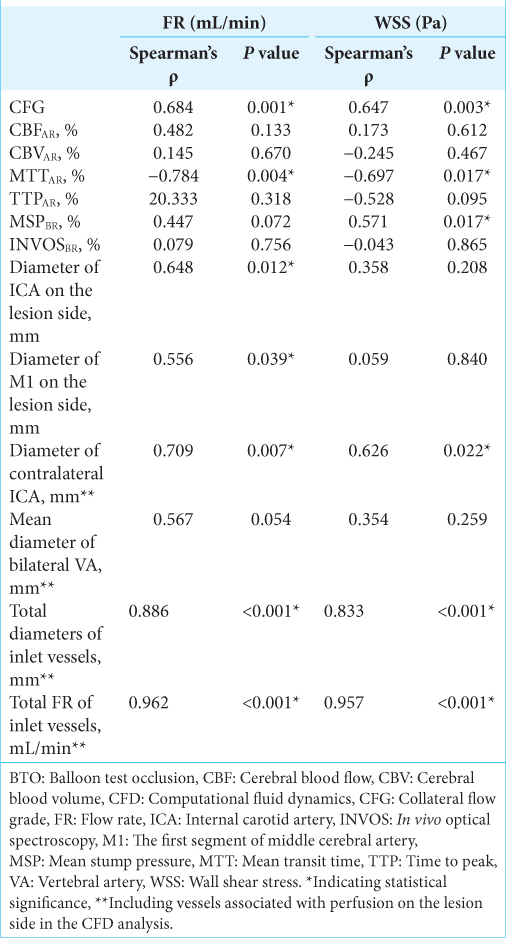
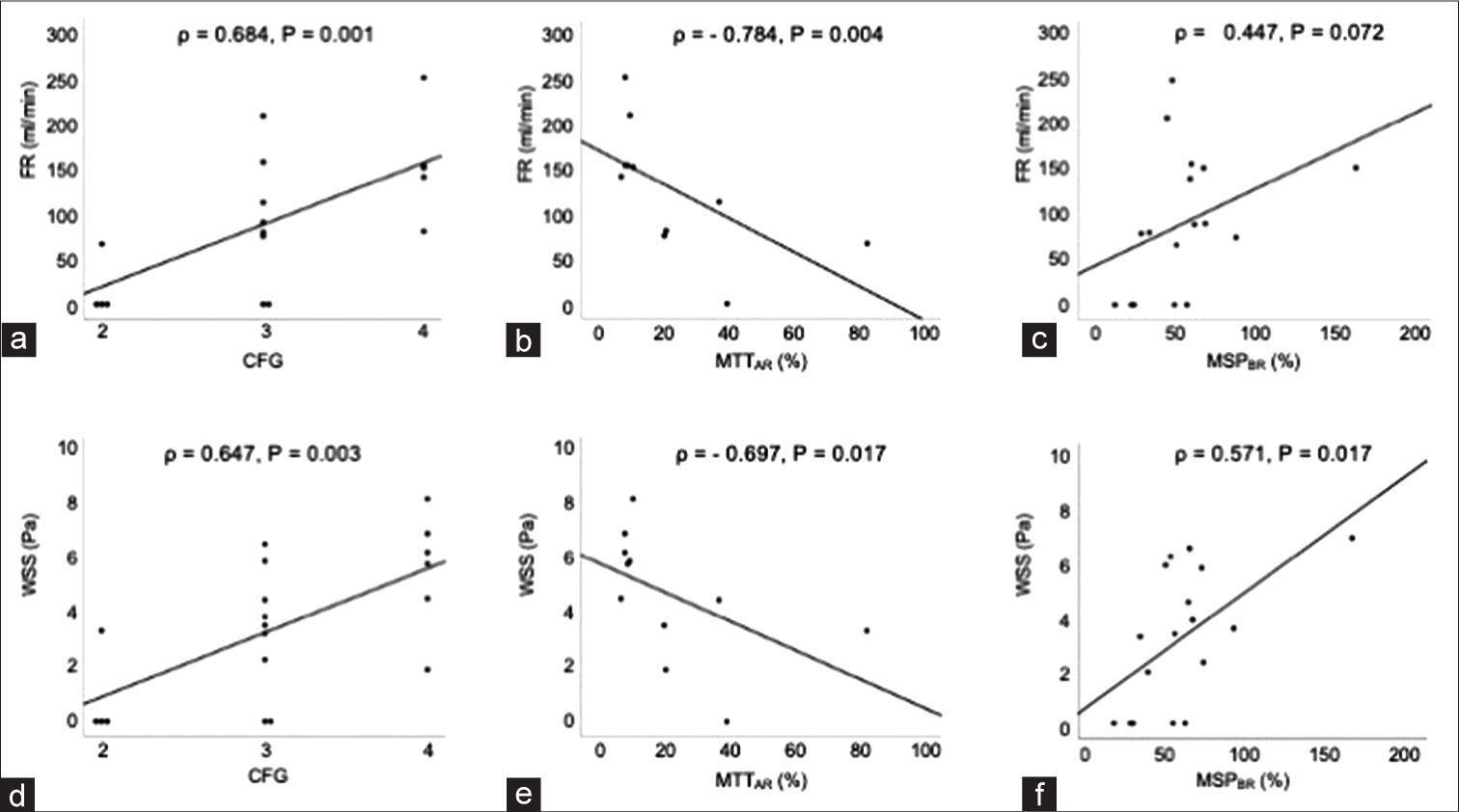
A case of left middle fossa tumor. Three-dimensional imaging data were obtained through CTA using a volume-rendering algorithm. (a) The white arrow shows the ICA on the lesion side. Extraction of the center lines and segmentation and labeling of the associated vessels were performed (b). ICA on the lesion side was set as a wall (white arrowhead), while that of the contralateral ICA and bilateral VA were set as the inlet, and the on-site analysis was run (c). After the analysis, the steam line contributing to M1 on the lesion side is shown (d). CTA: Computed tomography angiography, ICA: Internal carotid artery, M1: First segment of the middle cerebral artery, VA: vertebral artery.
In ZIOSTATION 2, the 3D image data were transformed to stereolithography (STL) format and Hemoscope Ver.1.5 (EBM Corp., Tokyo, Japan) was used for further image processing and analysis. In the vessel module, extraction of the center lines, segmentation, and labeling of the associating vessels (bilateral ICA, M1, A1, A2, VA, P1, P2, PcomA) were performed. These vascular geometries were filled with unstructured cells mainly consisting of hexahedrons approximately 0.25 mm in the far-wall regions and 0.125 mm (width) and 0.05 mm (height) in the near-wall regions. In the near-wall regions, the meshes were aligned to fit the boundary with three layers. The inlet (contralateral ICA, bilateral VA) and outlet vessels (bilateral M1, A2, P2) were extended as long as possible based on imaging data and trimmed their proximal and distal ends [
where Q, τ, µ, and D denote FR (mL/min), WSS (Pa), fluid viscosity, and vascular diameter, respectively. After total FR at the inflow vessels was calculated, the amount of FR was distributed at each outlet vessel according to equation;[
CFD analysis was performed using a finite volume method to solve the 3D unsteady Navier-Stokes equations and equation of continuity. Blood was assumed to be an incompressible and Newtonian fluid with a density of 1050 kg/m3 and a viscosity of 0.004 Pa s. The Euler and second-order upwind schemes were utilized to discern unsteady and convective acceleration terms. The convergent criteria were set to 10−4. Mean FR (mL/min) and mean WSS of the target vessels was obtained thorough this analysis, and the values (FR and WSS of M1 on the lesion side and FR of all inlet vessels) were evaluated. We named this simulation method virtual test occlusion. This method included vessels that were associated with perfusion on the lesion side. The virtual test occlusion with a steam line contributing to M1 on the lesion side is shown in [
Statistical analysis
The data are expressed as median (interquartile range). Spearman’s rank correlation coefficient was used to confirm the correlation between the parameters of virtual test occlusion and those of other modalities. A correlation coefficient (ρ) larger than 0.7, between 0.4 and 0.7, and between 0.2 and 0.4 indicated strong, moderate, and weak correlations, respectively. Receiver operating characteristic (ROC) curve analysis was used to determine the most suitable cutoff value of FR and WSS to detect good collateral groups based on the shortest distance from the curve to the upper left corner. Statistical analyses were performed using SPSS software (version 25; IBM Corporation, Armonk, NY, USA). P < 0.05 was considered statistically significant.
RESULTS
Correlations between parameters of virtual test occlusion and those of other modalities
Patient characteristics are shown in
A summary of the correlations is shown in
Figure 2:
Scatter diagrams showing the correlations between FR and collateral flow grade (a), MTTAR obtained through CT perfusion (b) and MSPBR (c), WSS and collateral flow grade (d), and MTTAR (e) and MSPBR (f). CT: Computed tomography, FR: Flow rate; AR: Asymmetry ratio, BR: Ratio before and after temporal occlusion, MTT: Mean transit time; MSP: Mean stump pressure, WSS: Wall share stress.
We subsequently evaluated the anatomical factors that affect the parameters of M1 on the lesion side obtained through virtual test occlusion. The FR of M1 on the lesion side showed strong correlations with the diameter of the contralateral ICA (ρ = 0.709, P = 0.007), total diameter of the inlet vessels (ρ = 0.886, P < 0.001), and total FR of the inlet vessels (ρ = 0.962, P < 0.001) and moderate correlations with ICA diameter on the lesion side (ρ = 0.648, P = 0.012), diameter of M1 on the lesion side (ρ = 0.556, P = 0.039), and mean diameter of the bilateral VA (ρ = 0.567, P = 0.054). WSS of M1 on the lesion side showed strong correlations with total diameter of the inlet vessels (ρ = 0.833, P < 0.001) and total FR of the inlet vessels (ρ = 0.957, P < 0.001) and a moderate correlation with contralateral ICA diameter (ρ = 0.626, P = 0.022) and weak correlations with ICA diameter on the lesion side (ρ = 0.358, P = 0.208) and mean diameter of the bilateral VA (ρ = 0.354, P = 0.259) with the exception of M1 diameter on the lesion side (ρ = 0.059, P = 0.840). Regarding anatomical factors, total FR of the inlet vessels was most strongly correlated with FR and WSS of M1 on the lesion side. It was also obtained through virtual test occlusion depending on anatomical information.
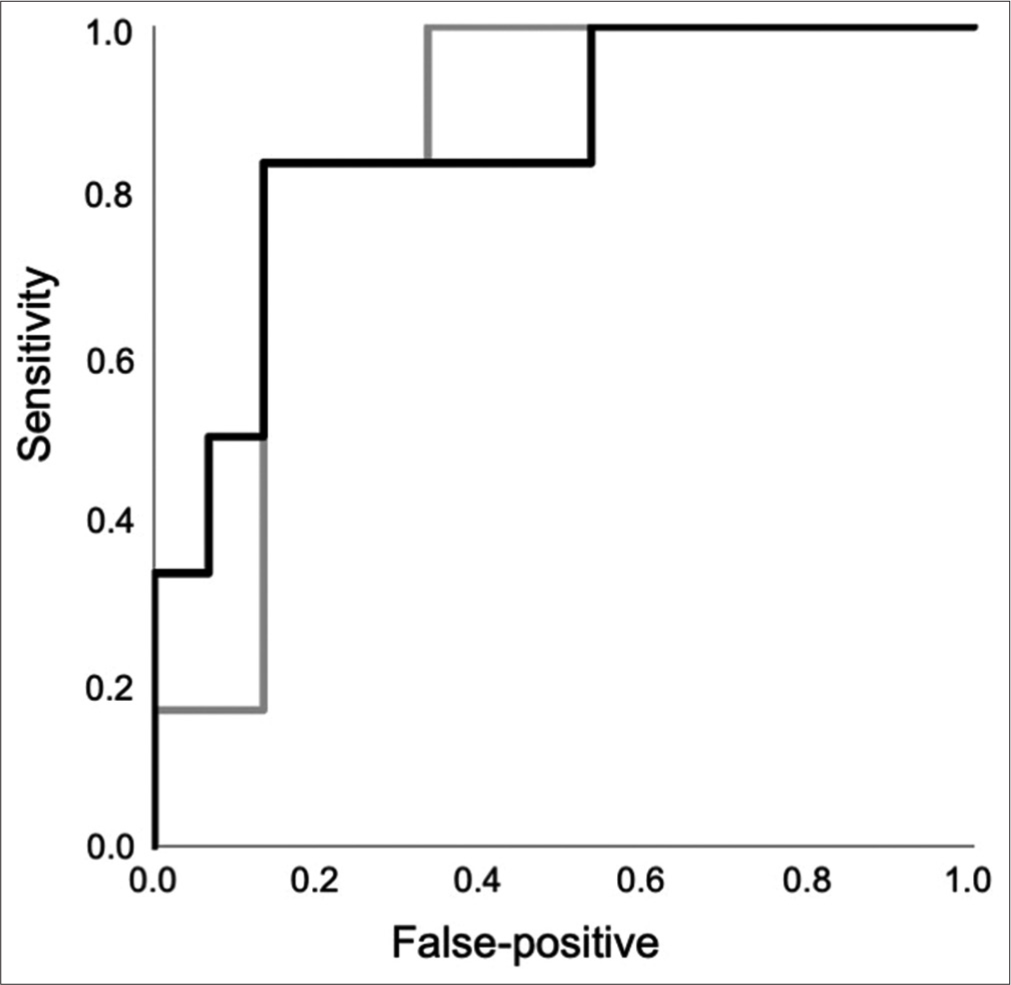
ROC curve analysis of good collateral group
The good collateral group consisted of six patients, while the poor collateral group consisted of 15 patients. The ROC curve analysis of FR and WSS of M1 on the lesion side to detect the good collateral group is shown in [
DISCUSSION
Utility of virtual test occlusion for assessing ischemia
In this study, the CFD parameters were correlated with collateral flow grade, MSP, and MTT. Collateral angiographic appearance, stump pressure, and MTT obtained through CT perfusion during BTO are reportedly predictors of ischemic tolerance,[
Regarding the items influencing CFD parameters, FR and WSS might be reflected, particularly inlet vessel diameter. FR obtained through CFD analysis reportedly reflects actual cerebral hemodynamic condition depending on anatomical structures. Kataoka et al. reported that vessel diameter and FR of intracranial arteries might be controlled so WSS remains constant after bypass surgery.[
Indications for recanalization with parent artery occlusion
In the present study, the most suitable cutoff values of FR and WSS for detecting the good collateral group obtained through ROC curve analysis were 126.2 mL/min and 4.54 Pa, respectively. These values represent the requirement for good collateral screening, and not indicating the need for revascularization surgery. The quantification of ischemic tolerance with FR and WSS obtained through virtual test occlusion using CFD analysis is expected to indicate ICA occlusion without high-flow bypass. However, the indications for revascularization surgery should be considered carefully with an appropriate safety margin to avoid delayed ischemic stroke. Regarding the requirement for revascularization surgery, various parameters using BTO have been reported previously. Abud et al. reported that carotid sacrifice without bypass was possible when the delay between the venous drainage of the injected and occluded hemisphere was <3 s.[
Limitations and future work
This study has several limitations. First, visualization of the small vessels such as the AcomA and PcomA was difficult because of partial volume effect and transformation to an STL format. The mean diameters of the AcomA and PcomA were reportedly 1.5 mm and 1.4–1.6 mm, respectively,[
CONCLUSION
Among the parameters obtained through CFD analysis, FR and WSS were correlated with collateral flow grade and MSP in addition to MTT. The good collateral group could be distinguished using virtual test occlusion and might be treated by ICA occlusion without high-flow bypass, although prospective validation is needed to confirm this. Most researchers focus on angiographic appearance and clinical data during BTO for detecting delayed ischemic stroke after parent artery occlusion, with little research having been done on non-invasively analyzing hemodynamic mechanisms. Virtual test occlusion might be used to evaluate ischemic tolerance as a non-invasive alternative to BTO.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Grant support: This work was supported in part by JSPS KAKENHI grant Number 20K15933 (to T.K.).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to thank Kei Miyata, Sangnyon Kim, Katsuya Komatsu, Yusuke Kimura, Rei Enatsu, and Yukinori Akiyama for helpful discussions.
References
1. Abud DG, Spelle L, Piotin M, Mounayer C, Vanzin JR, Moret J. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005. 26: 2602-9
2. Adams GL, Madison M, Remley K, Gapany M. Preoperative permanent balloon occlusion of internal carotid artery in patients with advanced head and neck squamous cell carcinoma. Laryngoscope. 1999. 109: 460-6
3. Brunberg JA, Frey KA, Horton JA, Deveikis JP, Ross DA, Koeppe RA. [15O]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 1994. 15: 725-32
4. Charbel FT, Zhao M, Amin-Hanjani S, Hoffman W, Du X, Clark ME. A patient-specific computer model to predict outcomes of the balloon occlusion test. J Neurosurg. 2004. 101: 977-88
5. Field M, Jungreis CA, Chengelis N, Kromer H, Kirby L, Yonas H. Symptomatic cavernous sinus aneurysms: Management and outcome after carotid occlusion and selective cerebral revascularization. AJNR Am J Neuroradiol. 2003. 24: 1200-7
6. Fox AJ, Vinuela F, Pelz DM, Peerless SJ, Ferguson GG, Drake CG. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987. 66: 40-6
7. Hashimoto A, Mikami T, Komatsu K, Noshiro S, Hirano T, Wanibuchi M. Assessment of hemodynamic compromise using computed tomography perfusion in combination with (123)I-IMP single-photon emission computed tomography without acetazolamide challenge test. J Stroke Cerebrovasc Dis. 2017. 26: 627-35
8. Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003. 34: e109-37
9. Huang Q, Xu J, Cheng J, Wang S, Wang K, Liu JM. Hemodynamic changes by flow diverters in rabbit aneurysm models: A computational fluid dynamic study based on microcomputed tomography reconstruction. Stroke. 2013. 44: 1936-41
10. Jou LD, Quick CM, Young WL, Lawton MT, Higashida R, Martin A. Computational approach to quantifying hemodynamic forces in giant cerebral aneurysms. AJNR Am J Neuroradiol. 2003. 24: 1804-10
11. Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuroda J. Treatment strategy for giant aneurysms in the cavernous portion of the internal carotid artery. Surg Neurol. 2007. 67: 148-55
12. Kataoka H, Makino Y, Takanishi K, Kimura Y, Takamura K, Yagi T. Vascular responses to abrupt blood flow change after bypass surgery for complex intracranial aneurysms. Acta Neurochir (Wien). 2018. 160: 1945-53
13. Khan MO, Arana VT, Rubbert C, Cornelius JF, Fischer I, Bostelmann R. Association between aneurysm hemodynamics and wall enhancement on 3D vessel wall MRI. J Neurosurg. 2021. 134: 565-75
14. Kuribara T, Mikami T, Iihoshi S, Miyata K, Kim S, Kawata Y. Ischemic tolerance evaluated by computed tomography perfusion during balloon test occlusion. J Stroke Cerebrovasc Dis. 2020. 29: 104807
15. Labeyrie MA, Lenck S, Bresson D, Desilles JP, Bisdorff A, Saint-Maurice JP. Parent artery occlusion in large, giant, or fusiform aneurysms of the carotid siphon: Clinical and imaging results. AJNR Am J Neuroradiol. 2015. 36: 140-5
16. Larson JJ, Tew JM, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: Long-term follow-up of 58 patients. Neurosurgery. 1995. 36: 26-30
17. Leng X, Lan L, Ip HL, Abrigo J, Scalzo F, Liu H. Hemodynamics and stroke risk in intracranial atherosclerotic disease. Ann Neurol. 2019. 85: 752-64
18. Linskey ME, Jungreis CA, Yonas H, Hirsch WL, Sekhar LN, Horton JA. Stroke risk after abrupt internal carotid artery sacrifice: Accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994. 15: 829-43
19. Mori F, Ishida F, Natori T, Miyazawa H, Kameda H, Harada T. Computational fluid dynamics analysis of lateral striate arteries in acute ischemic stroke using 7T high-resolution magnetic resonance angiography. J Stroke Cerebrovasc Dis. 2019. 28: 104339
20. Pedroza A, Dujovny M, Artero JC, Umansky F, Berman SK, Diaz FG. Microanatomy of the posterior communicating artery. Neurosurgery. 1987. 20: 228-35
21. Perlmutter D, Rhoton AL. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976. 45: 259-72
22. Ryu YH, Chung TS, Lee JD, Kim DI, Suh JH, Park CY. HMPAO SPECT to assess neurologic deficits during balloon test occlusion. J Nucl Med. 1996. 37: 551-4
23. Sanna M, Piazza P, Ditrapani G, Agarwal M. Management of the internal carotid artery in tumors of the lateral skull base: Preoperative permanent balloon occlusion without reconstruction. Otol Neurotol. 2004. 25: 998-1005
24. Satoh T, Yagi T, Onoda K, Kameda M, Sasaki T, Ichikawa T. Hemodynamic features of offending vessels at neurovascular contact in patients with trigeminal neuralgia and hemifacial spasm. J Neurosurg. 2019. 130: 1870-6
25. Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974. 41: 125-45
26. Shimizu K, Imamura H, Mineharu Y, Adachi H, Sakai C, Tani S. Endovascular parent-artery occlusion of large or giant unruptured internal carotid artery aneurysms. A long-term single-center experience. J Clin Neurosci. 2017. 37: 73-8
27. Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K. Magnitude and role of wall shear stress on cerebral aneurysm: Computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 2004. 35: 2500-5
28. Steinman DA, Milner JS, Norley CJ, Lownie SP, Holdsworth DW. Image-based computational simulation of flow dynamics in a giant intracranial aneurysm. AJNR Am J Neuroradiol. 2003. 24: 559-66
29. Suzuki T, Stapleton CJ, Koch MJ, Tanaka K, Fujimura S, Suzuki T. Decreased wall shear stress at high-pressure areas predicts the rupture point in ruptured intracranial aneurysms. J Neurosurg. 2019. 132: 1116-22
30. Takao H, Murayama Y, Otsuka S, Qian Y, Mohamed A, Masuda S. Hemodynamic differences between unruptured and ruptured intracranial aneurysms during observation. Stroke. 2012. 43: 1436-9
31. Takeda N, Fujita K, Katayama S, Tamaki N. Cerebral oximetry for the detection of cerebral ischemia during temporary carotid artery occlusion. Neurol Med Chir (Tokyo). 2000. 40: 557-62
32. Tani S, Imamura H, Asai K, Shimizu K, Adachi H, Tokunaga S. Comparison of practical methods in clinical sites for estimating cerebral blood flow during balloon test occlusion. J Neurosurg. 2019. 131: 1430-6
33. Tarr RW, Jungreis CA, Horton JA, Pentheny S, Sekhar LN, Sen C. Complications of preoperative balloon test occlusion of the internal carotid arteries: Experience in 300 cases. Skull Base Surg. 1991. 1: 240-4
34. Tomura N, Omachi K, Takahashi S, Sakuma I, Otani T, Watarai J. Comparison of technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission tomograph with stump pressure during the balloon occlusion test of the internal carotid artery. AJNR Am J Neuroradiol. 2005. 26: 1937-42
35. van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005. 26: 175-8
36. Vazquez-Anon V, Aymard A, Gobin YP, Casasco A, Ruffenacht D, Khayata MH. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: Technical aspects, cerebral monitoring, and results. Neuroradiology. 1992. 34: 245-51
37. Xiang J, Damiano RJ, Lin N, Snyder KV, Siddiqui AH, Levy EI. High-fidelity virtual stenting: modeling of flow diverter deployment for hemodynamic characterization of complex intracranial aneurysms. J Neurosurg. 2015. 123: 832-40
38. Zhu F, Qian Y, Xu B, Gu Y, Karunanithi K, Zhu W. Quantitative assessment of changes in hemodynamics of the internal carotid artery after bypass surgery for moyamoya disease. J Neurosurg. 2018. 129: 677-83