- Heaven-Gemini International Collaborative Group, Heaven Gemini Global Initiative, Turin, Italy.
Correspondence Address:
Sergio Canavero, Heaven-Gemini International Collaborative Group, Heaven Gemini Global Initiative, Turin, Italy.
DOI:10.25259/SNI_1130_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Canavero S. Whole brain transplantation in man: Technically feasible. Surg Neurol Int 23-Dec-2022;13:594
How to cite this URL: Canavero S. Whole brain transplantation in man: Technically feasible. Surg Neurol Int 23-Dec-2022;13:594. Available from: https://surgicalneurologyint.com/surgicalint-articles/12068/
The unavailability of technologies that can successfully rejuvenate an aged body suggests that it is time to explore other options. BRAVE, the BRain Anastomosis Venture[
Cephalosomatic anastomosis[
According to contemporary thinking, a full brain transfer from one living individual (Body Recipient, R) to another (Body Donor, D), a.k.a. cerebrosomatic anastomosis, is unachievable.
Four primary reasons are adduced: [
Impossibility to extract the brain proper from the dura mater, given the intimate relationship between the brain’s venous and cerebrospinal fluid (CSF) outflow and the dural cranial sinuses Impossibility to resuture the internal carotid and vertebral arteries (ICAs/VAs) and the internal jugular veins (IJV) once the brain is laid on the donor’s skull base Lack of an efficient technology to functionally reconnect the 12 pairs of cranial nerves Lack of a technology to reconnect the severed spinal cord Undetermined neuroprotective measures to deploy between the moment of physical separation of the brain from R’s skull and re-establishment of circulation after positioning on D’s skull base Possible immune rejection if BT is carried out on a heterologous body rather than R’s clone.
The last three points are covered elsewhere. In particular, the spinal cord – once sharply severed – can be functionally reconnected in primates (GEMINI protocol: Fully reviewed in Canavero and Ren;[
In this paper, I summarily address the first three points in that order. More technical details and extensive discussion and referencing are found elsewhere.[
THE BRAIN IS TRANSPLANTED ALONG WITH THE DURAL SAC
Sparing the dural venous sinuses along with all the veins and arachnoidal granulations is currently unachievable. Besides, the subdural circulation of CSF would be totally disrupted. The solution is transplanting the brain inside the dural sac.
Briefly, both individuals are trachetomized and ventilated and installed in the upright position. Heads are secured on both sides with a fixation apparatus adapted from the maxillofacial equivalent[
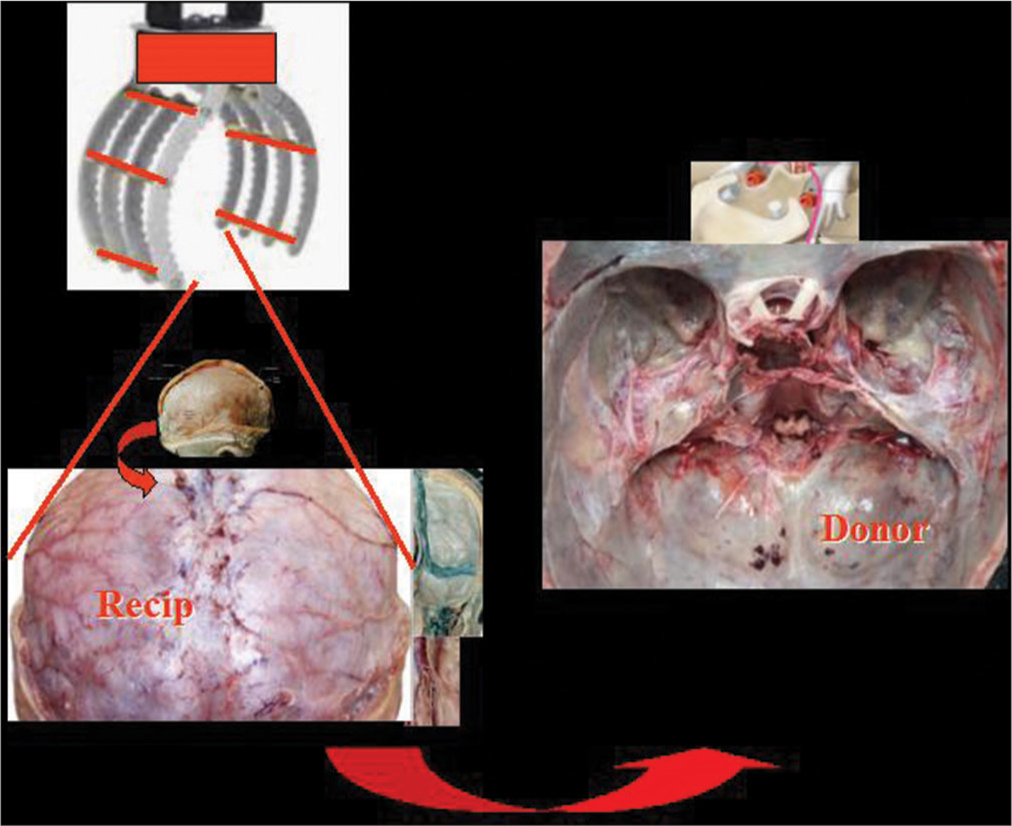
Predicated on the complete expendability of R’s body, the approach in R starts with a nasion-C7 spinous process linear incision followed by full thickness scalping of the head down to the orbital ridges. The skull cap is removed in standard fashion, with multiple burr holes on the two sides of the superior longitudinal sinus and other holes, including along the basal circumference. A standard wide craniectomy frees the cerebellum [
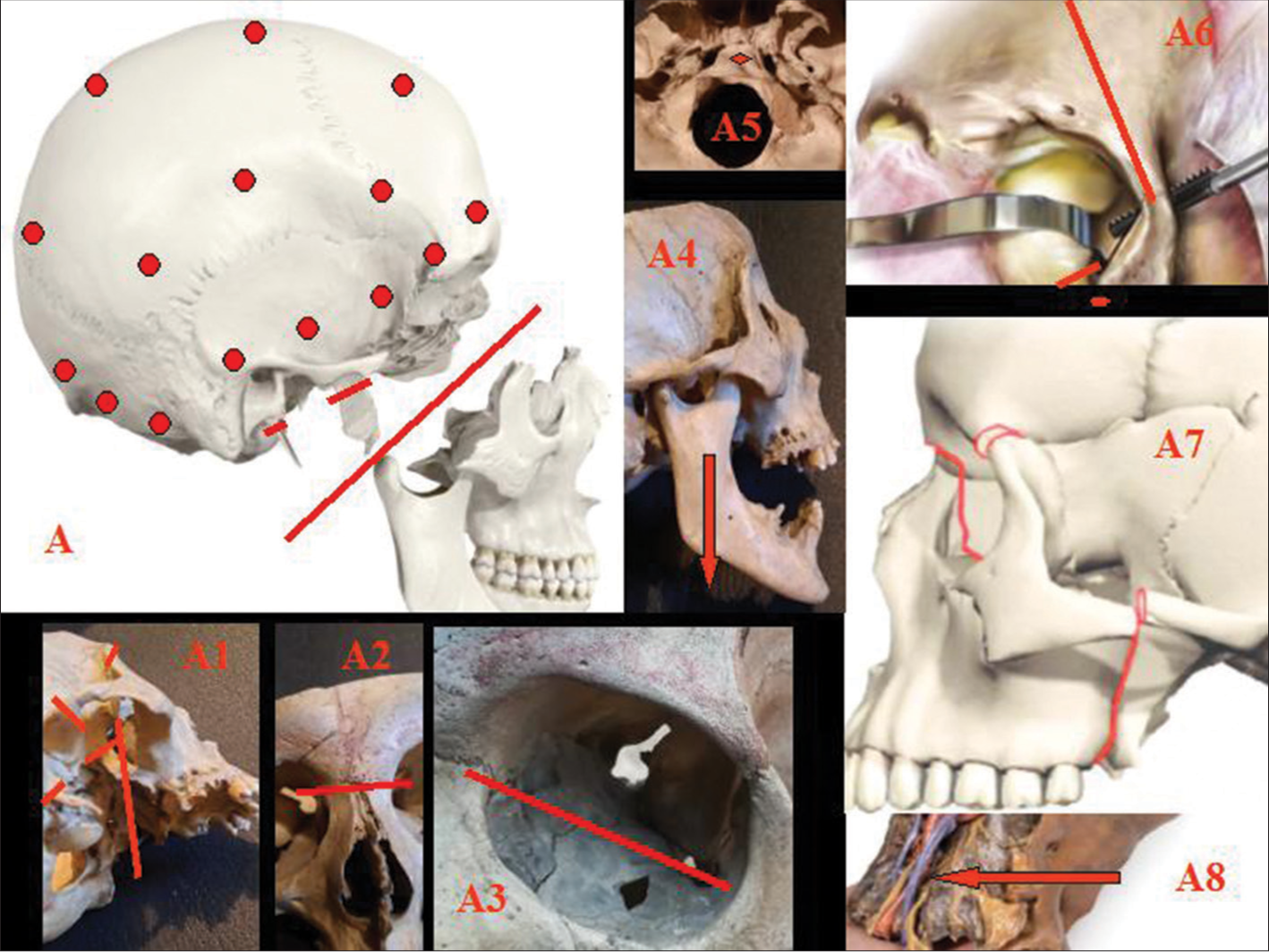
Figure 1:
A: Burr holes (red circles) for skull detachment from dura; splitting of splanchnocranium (red line); removal of pterygoid process; and styloid process (red segments). A1-4: LeFort III osteotomy (red lines). A5: The clivus is removed (red circle). A6: Orbital step of LeFort III osteotomy (red lines). A7: Lines of fracture for full splanchno-cranium detachment (red lines). A8: Unrestricted view of the neurovascular bundle in the neck that can be followed up to cranial entry (arrow).
A slightly modified LeFort III osteotomy follows.[
In D, a standard coronal incision is followed by a linear incision from the bregma through the inion down to C7 (T incision). The frontal skin flap is rolled forward in classic fashion, while the two hemihalves of the posterior teguments are retracted sideways. The skull cap is removed en bloc after a single linear high-speed saw cut through the skull bone proper along its basal circumference and set aside for repositioning at the end of the surgery. D’s brain can be removed en bloc (hypophysis included) along with the hemispheric dural sac and the upper metameres of the cervical cord, without fear of damage. However, the basal dura is left in place, as this will not interfere with the whole process. Bilateral anterior and posterior clinoidectomies are carried out to facilitate later hypophyseal repositioning, nerve fusion, and vascular anastomosis in the sella turcica region.
In both R and D, the cervical spinal cord is accessed in standard fashion with removal of the C1-5 spinous processes (in D, these are repositioned after GEMINI spinal cord fusion). However, longitudinal durotomy is delayed until the final process of spinal fusion.
In both R and D, the ICA is severed above the point of origin of the ophthalmic artery (whose branches include the central retinal artery) and temporarily clamped. The IJV is severed distally as it emerges from its bulb. Cranial nerves are severed beyond the points of later actual fusion, so as to have fresh interfaces after further trimming.
As R’s brain encased in its dura (hypophysis included) is being freed from the cranial vault and base, a robotic scoop with retractable tines is brought into the operating field. This envelops the brain and supports it as the dural detachment proceeds, and facilitates the final transfer onto D [
It goes without saying that the surgeries in R and D must be synchronized so that transfer of R’s brain can occur even as D’s brain is removed.
In sum, BT should be understood as a meningo-cerebro-somatic anastomosis.
VASCULAR RECONNECTION
BRAVE requires a rapid restart of the circulation to R’s brain. However, anatomical (in particular, the highly constrained surgical space of the cranial fossae) and technical considerations[
A pretransplant angiography in both R and D is mandatory to assess the entire vasculature, including anatomic variations (e.g., absence/hypoplasia of the ICA and IJV) and size mismatches.[
Briefly, the ICA enters the skull through the carotid canal (diameter in adults: 6–7 mm on the right, 6–8 mm on the left) along with the venous plexus and veins surrounding it and the sympathetic nerves. The ICA leaves the cavernous sinus before and medial to the anterior clinoid. The subarachnoid segment has a width of 2.8–3.3 mm and is 13.4 (8–18) mm long; it then bifurcates into the middle and anterior cerebral arteries. The IJV’s diameter is 9.1–10.2 mm, but in a minority, it can be <5 mm. The VA – with the left being dominant – has a mean diameter (C3 level) of 3.5–3.6 mm in males and 3–3.4 mm in females.[
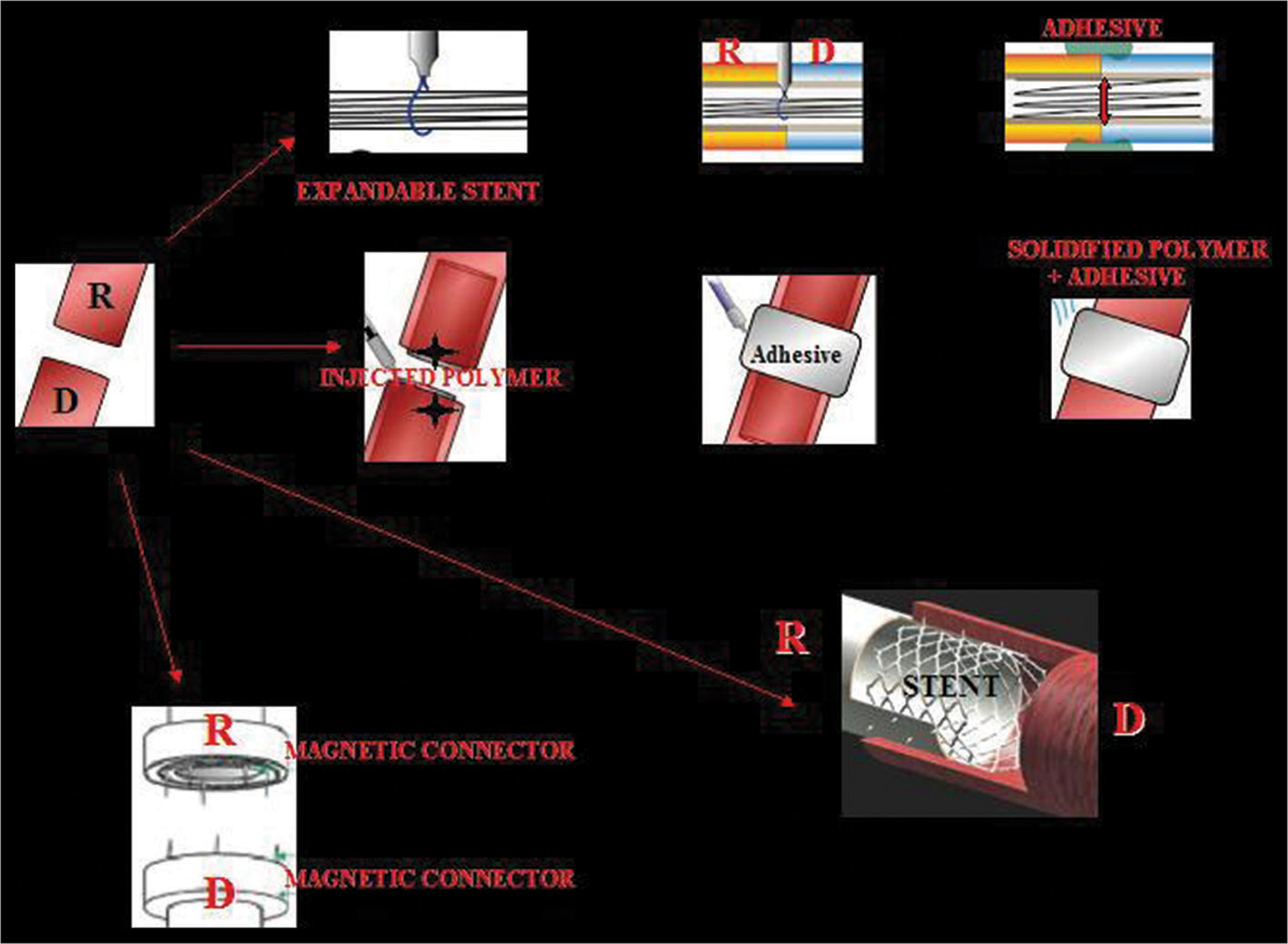
Several scenarios are possible, including stent-assisted vascular anastomosis (SAVATOM) and Magnetic Anastomosis (MAGSTOM) [
Figure 3:
The vessels (here are shown the carotids) of both recipient (R) and donor (D) can be anastomosed with sutureless vascular anastomosis technology. Four options are illustrated: 1 – an expandable stent can be inserted in both stumps, the stumps are then approximated, the stent is fully expanded; an adhesive adds stability to the coaptation (stent-assisted vascular anastomosis, SAVATOM 1); 2 – a polymer can be injected into the lumen of the vessels; an adhesive stabilizes the complex while the polymer solidifies (SAVATOM 2); 3 – a nonresorbable stent with pins, once deployed, effects the anastomosis (SAVATOM 3); 4 – magnetic connectors inserted into the two stumps ensure immediate coaptation Magnetic Anastomosis. 3D printing is ideal for personalized stents (From Canavero and Ren,[
CRANIAL NERVE RECONNECTION
Similarly to vascular reconnection, sutured anastomosis is ruled out for cranial nerve reconstruction. Besides the constrained operative space, microsuturing cannot reestablish cranial nerve function rapidly, being exclusively dependent on regeneration. In addition, microsuturing is traumatizing to the nerve (reviewed in De Medinaceli[
Importantly, R’s and D’s cranial nerves are severed beyond the actual point of final connection so that any Wallerian degeneration that usually starts within minutes of section is offset by severing the initially degenerating extra-length once the brain has been placed on D’s skull base and fusion initiated (see in Canavero and Ren[
Rodent studies show that, at least for healthy sciatic nerves, stretching up to 30% is not harmful: this is important for surgical maneuvering.[
All cranial nerves have <100,000 axons each, with the optic nerve being the outlier (1,200,000 fibers).
Fusion exploits so-called fusogens, such as PolyEthylene Glycol (PEG) and chitosan (reviewed in Ryan and Henderson[
Multiple approaches enable nerve approximation, including Photochemical Tissue Bonding and MAGSTOM.[
A caveat should be added. Unless performed in a clone, variation of the diameter of cranial nerves must be assessed with high-resolution MRI before BT in D.
It is worth mentioning how the entire surgery might be adapted so that the eyes are also transplanted without damaging the neurovascular bundle. However, the eyes also degenerate with age.[
CONCLUSION
Contrary to common lore, a full BT is achievable, at least theoretically. Of course, further extensive cadaveric rehearsals will be necessary, followed by tests in brain-dead organ donors (as e.g., done recently in kidney xenotransplants). New surgical tools will have to be developed. With appropriate funding, a long-held dream may finally come true.[
Commentary
Kidney, Liver, Lung, Hand, Heart, and Face Transplantation. Brain transplantation?
This concept of a brain transplant is an advanced plan based on Dr. Canavero’s previous experience with acute spinal cord repair which has been fully confirmed to succeed in animals from rats to cats, dogs, and monkeys. That work was never believed possible until Dr. Canavero assembled a team of scientists worldwide to solve the many aspects of that achievement. The work was based on a fundamental misunderstanding of the mechanisms involved in spinal neuron regrowth. His revelation that the short neurons regrow to re-establish primitive motor pathways present in species from their origins is a breakthrough in science.
Now, Dr. Canavero is challenging science and humanity with the possibility of a brain transplant. Kidney, liver, lung, hand, heart, and face transplants have all been done in the late 20th century. Yet, the scientific success was delayed in these major advances by the emotional, ethical, and religious objections which were raised. This project of a brain transplant will not doubt undergo similar criticism. Yet, the scientific question remains as to whether such a transplant can be achieved. That is the major focus of Dr. Canavero’s work.
Humans have discovered the manipulation of atoms and molecules which can be used for the good or bad purposes in benefitting civilizations, as in work with fusion and fission and the development of atomic weapons that can kill millions of people or produce energy benefiting all. That is a human problem in the use of knowledge with good or evil intent which extends to many aspects of life. Prejudgment of the experiments misses the point of this work, as it did with the discovery that the spinal cord can be repaired, which Dr. Canavero and his colleagues proved. The ultimate goal was to reestablish a functional life.
This brain transplant program is also complex, the results of which are not known to questions not yet asked. There are many aspects of the giant project which Dr. Canavero is considering which will need to be tested and validated. However, assume for a moment that the project is successfully done. What will we learn? Can a brain of a nonathletic person be transplanted to an athletic one and how will that brain function? What reorganization will we see? What further secrets to an understanding of the human body will emerge for us to learn more about the brain-body interactions? This work can only be fully understood in its use in humans after its validation in animals. Can a person born without arms undergo regeneration of those appendages? That does happen in animals. Imagine the impact of such a discovery on humans now and in the future.
In science, asking the proper questions leads to more knowledge of the solution of disease states and longevity. Already, as a result of the advances in science, the life expectancy of humans has tripled in the past 150 years, a monumental accomplishment of humans. Such ideas challenge our traditional views and should be accepted and properly tested. To reject questions on the basis of traditional or emotional views will only stop the advances in science and an understanding of our place in the universe and inhibit our progress as a civilization in the future in all fields.
James I. Ausman, MD, MA, PhD
Professor, Neurosurgery UCLA
CEO of Surgical Neurology International® and SNI Digital™, Worldwide 21st Century methods of Scientific Communication among people.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Canavero S. Total eye transplantation for the blind: A challenge for the future. Med Hypotheses. 1992. 39: 201-11
2. Canavero S, Ren XP, editors. The Technology of Head Transplantation. New York: Nova Science; 2020. p.
3. Canavero S. The Technology of Brain Transplantation. Available from: https://www.amazon.com/technology-brain-transplantation-sergio-canavero/dp/b08bdwy9q2 [Last accessed on 2022 Dec 01].
4. Canavero S, Ren XP, Kim CY. Heterologous spinal cord transplantation in man. Surg Neurol Int. 2021. 12: 295
5. De Medinaceli L, editors. Cell Surgery to Repair Divided Nerves. New York: C.A.S.I.S; 1994. p.
6. Ren XP, Gkasdaris G, Canavero S, Canavero S, Ren XP, editors. Brain protection during cephalosomatic anastomosis. The Technology of Head Transplantation. New York: Nova Science; 2020. p. 113-22
7. Ren XP, Henderson P, Kim CY, Canavero S, Rajendram R, Preedy VR, Martin CR, editors. GEMINI-supported spinal cord transplantation for the treatment of chronic spinal paralysis: overview and initial clinical translation. The Diagnosis and Treatment of Spinal Cord Injury. The Neuroscience of Spinal Cord Injury. London: Academic Press Elsevier; 2022. p. 313-24
8. Ryan I, Henderson PW, Canavero S, Ren XP, editors. GEMINI: Overview of fusogens. The Technology of Head Transplantation. New York: Nova Science; 2020. p. 51-68
9. Schlieder D, Markiewicz MR. Craniofacial syndromes: The Le Fort III osteotomy for correction of severe midface hypoplasia. Atlas Oral Maxillofac Surg Clin North Am. 2022. 30: 85-99
10. Zhavoronkov A. Can You Transplant a Brain into a Young New Body? And would you? FORBES. Available from: https://www.forbes.com/sites/alexzhavoronkov/2022/11/22/canyou-transplant-a-brain-into-a-young-new-body-and-would-you/?sh=588594983d2c [Last accessed on 2022 Dec 01].