- Theoretical Neuroscience Research, LLC, Ridgeland, Mississippi, United States.
Correspondence Address:
Russell L. Blaylock, Retired Neurosurgeon, Theoretical Neuroscience Research, LLC, Ridgeland, Mississippi, United States.
DOI:10.25259/SNI_626_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Blaylock RL. Why immunoexcitoxicity is the basis of most neurodegenerative diseases and systemic immune activation: An analysis. Surg Neurol Int 04-Aug-2023;14:281
How to cite this URL: Blaylock RL. Why immunoexcitoxicity is the basis of most neurodegenerative diseases and systemic immune activation: An analysis. Surg Neurol Int 04-Aug-2023;14:281. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12478
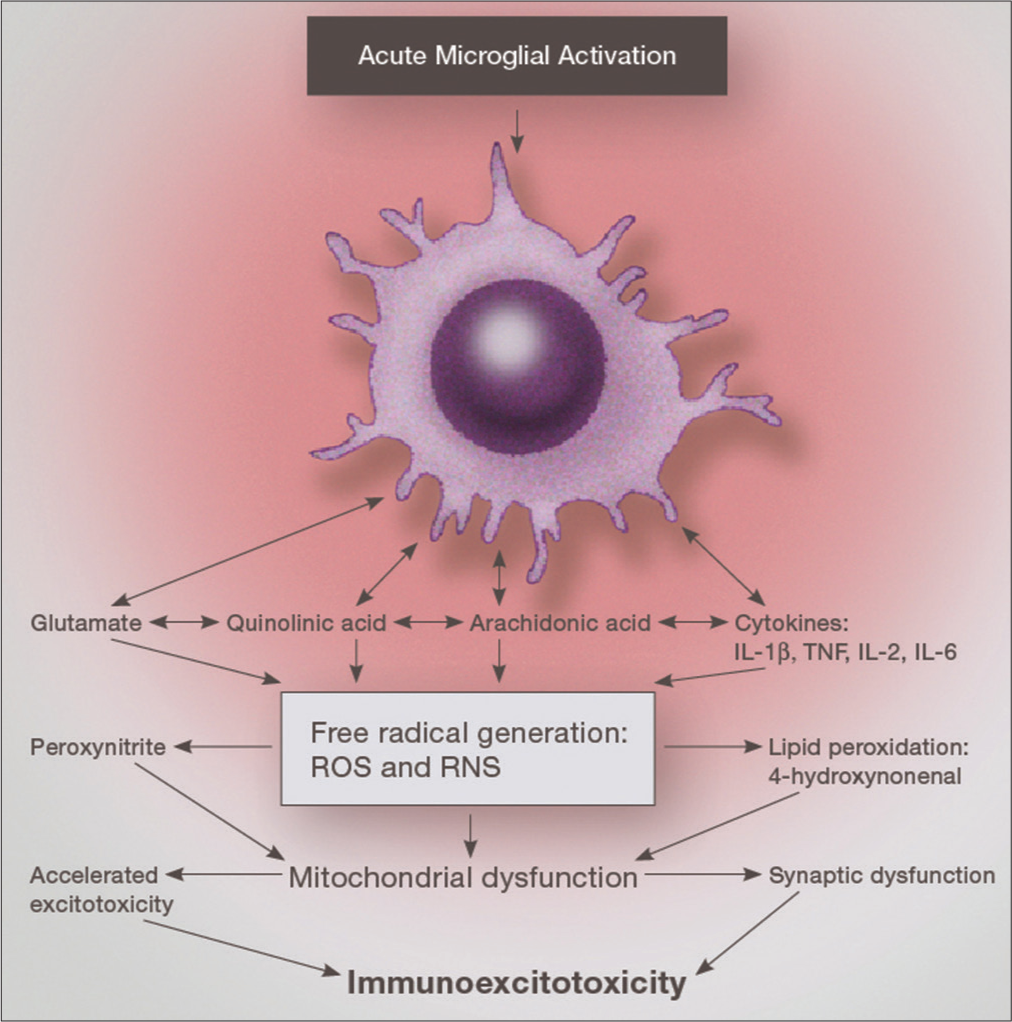
Immunoexcitotoxicity simply means a connection between immune activation in the body and enhancement of excitotoxicity in tissues containing glutamate receptors. This series of reactions occurs principally by the number of systems at play. It has been demonstrated, for example, that the glutamate transporters are inhibited by reactive oxygen species (ROS) as well as activation of cell systems that make up the various glutamate (excitotoxic) receptors, basically their subunits.[
In the first instance, ROS are known to inactivate the main glutamate transporters, GLT1, and GLAST.[
In most cases, the most destructive excitotoxic reaction occurs by opening a cell membrane calcium pore.[
In the immunoexcitotoxic reaction, ROS are massively generated and consequently the transporters are inactivated [
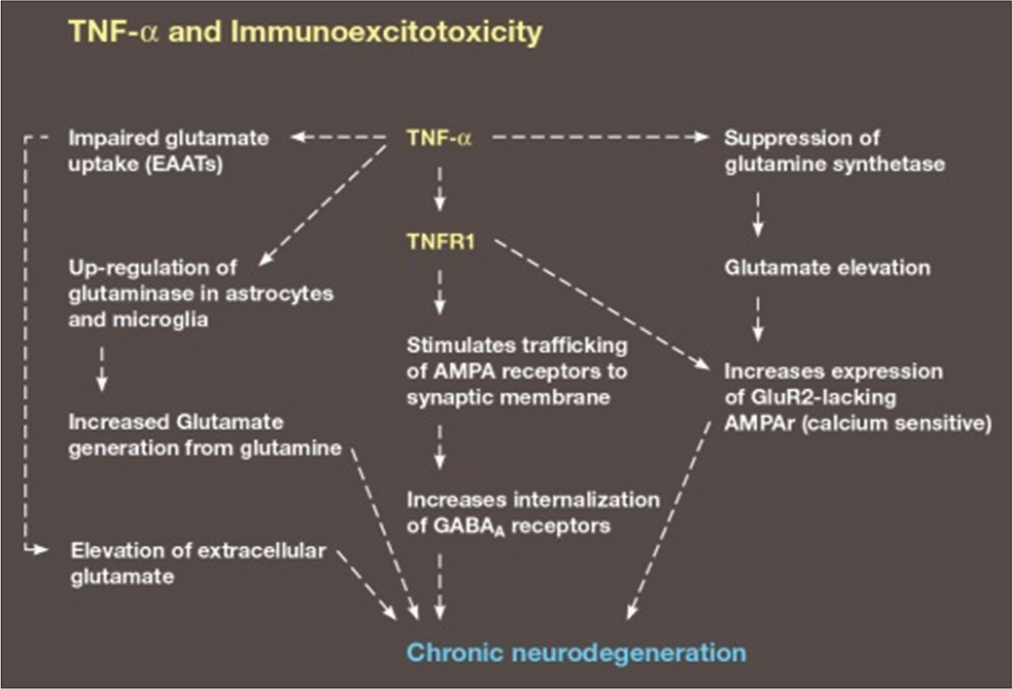
Figure 1:
Demonstration of various mechanisms used by the immune system to enhance excitotoxicity. EAATs: Excitatory Amino Acid Transporters, TNFα:Tumor Necrosis Factor alpha, TNFR1:Tumor necrosis factor receptor 1, AMPA:α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, (GABAA):γ-Aminobutyric acid type A (GABAA).
Inflammatory cytokines generate several free radicals (especially interleukin-1 beta [IL-1ß] and tumor necrosis factor-alpha [TNF-alpha]). A second reaction that has been recently recognized is the ability of some inflammatory cytokines (IL-1ß and TNF-alpha) to enhance particular excitotoxic subunits, for example, the NR1 subunit of the N-methyl-D-aspartate (NMDA) receptor [
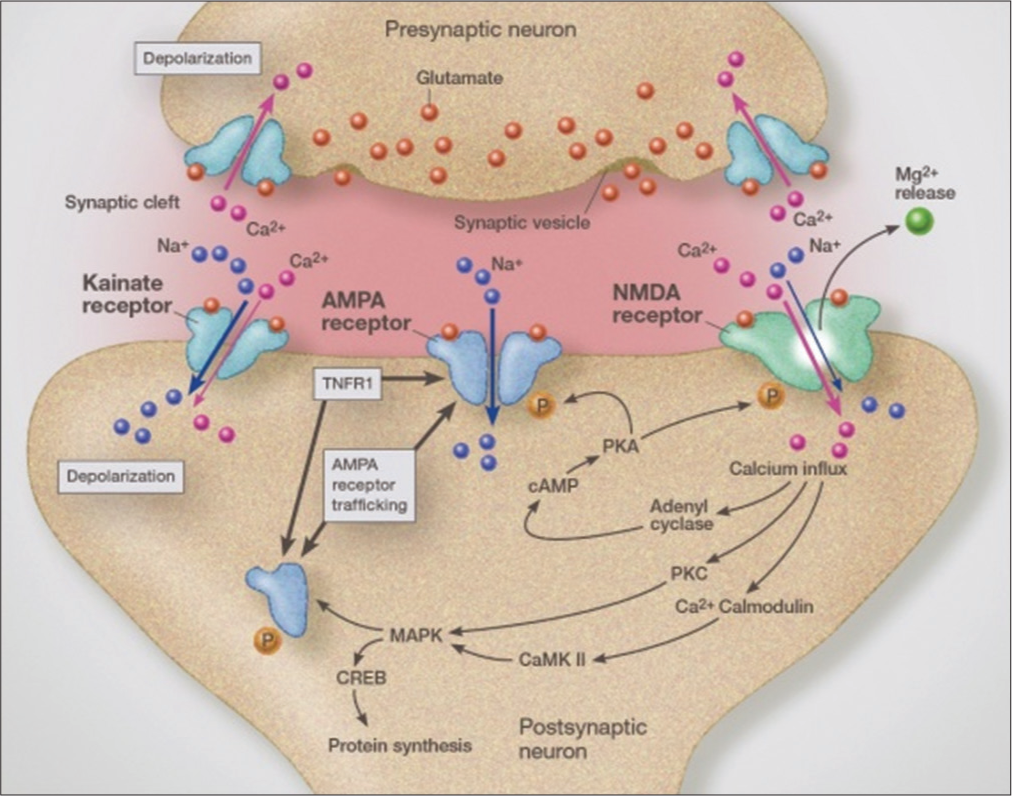
Figure 2:
Illustration demonstrating the various glutamate receptors and the effect of activating TNFR1 and well as calcium channels in this process. NMDA: N-methyl-D-aspartate, PKA: cAMP-dependent protein kinase A, PKC: Protein kinase C, cAMP: Cyclic adenosine monophosphate, MAPK:Mitogen-activated protein kinase, CREB: cAMP-response element binding protein, CaMkII: Ca2+/calmodulin-dependent protein kinase-II.
Like NMDA receptors, they are calcium permeable and are responsible for greatly enhanced excitotoxicity. The AMPA receptor normally makes up the fast transmission system. With inflammation, anywhere in the body, they become more destructive within the CNS. Unlike NMDA receptors, they are not controlled by magnesium.[
The metabotropic receptors control the sensitivity of the main glutamate receptors, (NMDAR, AMPAR, and kainate receptors). By enhancing the sensitivity of metabotropic receptor 1 (an activator), the inflammatory cytokines can enhance the sensitivity of the main receptors, especially NMDA receptors [
There are other systems at play in excitotoxicity, such as the Xc system, which exchanges external cystine for internal glutamate.[
Recent research indicates that there are hemichannels that move glutamate out of the cell in massive amounts and that inflammatory cytokines can activate these hemichannels worsening excitotoxicity.[
In addition, TNF-alpha suppresses glutamine synthetase, an enzyme which protects the neuron by converting glutamate to glutamine [
In essence, we see a very intimate connection between glutamate receptors and the immune system mediators. It has been shown that this enhancement is present even with minor surgical operations systemically. The length of this enhancement varies from a few days to decades (in the case of head trauma and autism).[
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Blaylock RL, editors. The Danger of Excessive Vaccination during Brain Development: The Case for a Link to Autism Spectrum Disorders (ASD). Luxembourg. MedVeritas. 2008. p. 1727-41
2. Bridges RJ, Natale NR, Patel SA. System xc⁻ cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012. 165: 20-34
3. Christ M, Müller T, Bien C, Hagen T, Naumann M, Bayas A. Autoimmune encephalitis associated with antibodies against the metabotropic glutamate receptor Type 1: Case report and review of the literature. Ther Adv Neurol Disord. 2019. 12: 1756286419847418
4. Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987. 8: 329-43
5. Godoy JA, Rios JA, Picon-Pages P, Herrera-Fernandez V, Swaby B, Crepin G. Metastasis, calcium and free radicals in health, aging and neurodegeneration. Biomolecules. 2021. 11: 1012
6. Guo C, Ma YY. Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders. Front Neural Circuits. 2021. 15: 711564
7. Hanly JG, Robichaud J, Fisk JD. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006. 33: 1553-8
8. Kopach O, Dobropolska Y, Belan P, Voitenko N. Ca(2+)-Permeable AMPA receptors contribute to changed dorsal horn neuronal firing and inflammatory pain. Int J Mol Sci. 2023. 24: 2341
9. Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007. 6: 337-50
10. Matute C, Torre I, Perez-Cerda F, Samartin A, Alberdi E, Etxbarria E. P2x7 receptor blockade prevents excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalitis. J Neurosci. 2007. 27: 9525-33
11. Perry VH. The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav Immun. 2004. 18: 407-13
12. Raffaello A, Mammucari C, Gherardi G, Rizzuto R. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci. 2016. 41: 1035-49
13. Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008. 43: 840-6
14. Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med (Berl). 2000. 78: 3-13
15. Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006. 281: 21362-8
16. Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Ann Neurol. 2003. 53: 588-95
17. Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007. 85: 2059-70
18. Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005. 57: 67-81
19. Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994. 14: 2924-32
20. Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008. 154: 1533-8
21. Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S. Activated mast cells contribute to postoperative cognitive dysfunction by evoking microglial activation and neuronal apoptosis. J Neuroinflam. 2016. 13: 127






Dr. Miguel A. Faria
Posted August 5, 2023, 1:13 pm
This unifying biological theory also explains, both directly and indirectly, why inflammation plays a detrimental role associated with increased cancer rates, decreased beneficial immunity, neurodegeneration, as well as increased aging. Agents that decrease these deleterious effects may be effective in clinical practice. Dr. Blaylock has done a magnificent job summarizing why immunoexcitotoxicity is the basis for neurodegeneration and other pathophysiological changes in the CNS.