- Department of Neurosurgery, Memfys Hospital, Enugu, Nigeria.
- Department of Radiology, Memfys Hospital and University of Nigeria, Enugu, Nigeria.
- Department of Neurology, Memfys Hospital, Enugu, Nigeria.
- Department of Neurology, University of Nigeria and Memfys Hospital, Enugu, Nigeria.
Correspondence Address:
Samuel Chukwunonyerem Ohaegbulam, Department of Neurosurgery, Memfys Hospital, Enugu, Nigeria.
DOI:10.25259/SNI_136_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel Chukwunonyerem Ohaegbulam1, Chika Anele Ndubuisi1, Okwunodulu Okwuoma1, Wilfred Mezue1, Enyereibe Chuks Ajare2, Bibiana Oti3, Sunday Achebe1, Francis Campbell1, Donald Ogolo1, Birinus Ezeala-Adikaibe4. Will improved neuroradiology facilities debunk the reported rarity of intracranial aneurysms in Sub-Saharan Africa?. 31-Mar-2023;14:113
How to cite this URL: Samuel Chukwunonyerem Ohaegbulam1, Chika Anele Ndubuisi1, Okwunodulu Okwuoma1, Wilfred Mezue1, Enyereibe Chuks Ajare2, Bibiana Oti3, Sunday Achebe1, Francis Campbell1, Donald Ogolo1, Birinus Ezeala-Adikaibe4. Will improved neuroradiology facilities debunk the reported rarity of intracranial aneurysms in Sub-Saharan Africa?. 31-Mar-2023;14:113. Available from: https://surgicalneurologyint.com/surgicalint-articles/12229/
Abstract
Background: Intracranial aneurysms (IAN) are rare in the Sub-Saharan Africa unlike other parts of the world. The debate is whether the low frequency might be apparent because of the scarcity of advanced neuroimaging services, or real. This study investigated if improved imaging facilities would debunk the rarity of IAN in our subregion.
Methods: This is a retrospective cohort study of prospectively recorded data of patients with subarachnoid hemorrhage (SAH) and IAN managed over 19 years (2003–2021), at the study center with a catchment population of over 47 million. The center witnessed progressive improvements in neuroimaging facilities: 2-Slice, 8-slice, and 64-slice computed tomography (CT) and 0.35T, 1.5T magnetic resonance imaging (MRI) during the period.
Results: There were 241 cases of SAH, but only 166 aneurysms were confirmed in 158 patients. Between 2003 and 2008, only 27 IAN patients (4.5 IAN/year) were diagnosed. After introduction of CT angiography/magnetic resonance angiography MRA using 8-slice CT/0.35T magnetic resonance imaging (MRI), between 2009 and 2014, the frequency of IAN increased to 8/year. Between 2015 and 2018 after installation of a 64-slice CT in 2014, the IAN remained the same (8/year). MRI 1.5T was added in 2018, the frequency doubled to 17 cases/year. The females were more (67.7%), the mean age was 46.3 years, but peak incidence was the sixth decade. Internal carotid artery aneurysms including posterior communicating artery were the most common (43%) followed by ACA with anterior communicating artery (24%) and middle cerebral artery (20%). Multiple aneurysms were seen in ten patients.
Conclusion: Improved neuroimaging between 2003 and 2021 did not debunk the rarity of IAN in our region.
Keywords: Africa, Geographical neurosurgery, Intracranial aneurysm epidemiology, Subarachnoid hemorrhage
INTRODUCTION
Sub-Saharan Africa has consistently recorded low intracranial aneurysm (IAN) frequencies prompting some reporters to argue that IAN are rare.[
Mortality and morbidity from aneurysmal subarachnoid hemorrhage (aSAH) remain high despite immense research work and improvements in diagnostic and therapeutic capabilities. Each year, approximately 1 in 10,000 North Americans suffer aSAH, and despite recent advances, morbidity and mortality remain high as about 50% of aSAH patients die from the first hemorrhage or later complications.[
There is documented geographical variation in the incidence and frequency of IANs. The incidence rate of aneurysmal SAH in developed countries varies from 1% to 5% of the population. A low incidence has been reported from some parts of Asia and Africa.[
Studies such as ours are important and needed to better understand the epidemiologic, clinical, and economic burden of cerebral aneurysms in Africa at national and regional levels. The study of the epidemiology of IANs may identify populations with a high or low risk of developing IANs and therefore facilitate the identification of genetic or acquired factors that might be responsible and thus drive prevention strategies.
The aim of this study was to review the annual frequency of IANs seen at a major referral neurosciences hospital in South-Eastern Nigeria over a 19-year period (2003–2021) that included a period of progressive upgrade of diagnostic facilities. Have the improved diagnostic facilities and awareness led to increase in the frequency of diagnosed IANs in the center?
MATERIALS AND METHODS
This is a retrospective analysis of prospectively recorded data of patients with the diagnosis of IAN seen between January 1, 2003, and December 31, 2021, at the study center. Information was obtained from clinic records, radiology (angiography, computed tomography [CT], and magnetic resonance imaging [MRI]), inpatient and operating theater database of the hospital.
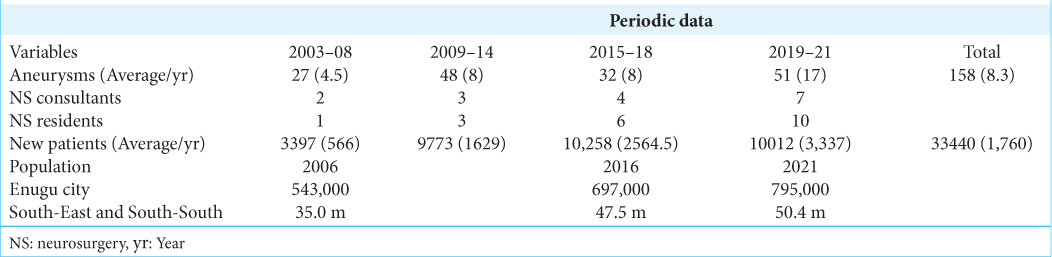
The study was carried out in a private tertiary hospital established in August 2002. It was the only hospital that provided surgical management of IAN cases in the sub-region during most of the period of the study. The center had 8 consultant neurosurgeons and 30 Senior Neurosurgical Residents over the period of the study. Although located in a city with a population of about 723,000 (2006 National Census), the hospital also served as a referral center for many states, providing cerebral angiography (CA), CT angiography (CTA) and MRA services. The catchment population of mixed socio-economic status is over 47 million [
Diagnosis of aSAH was based on history, examination, lumbar puncture, carotid angiography, CTA, and/or MRA. The protocol of the hospital for aSAH included repeating the CTA 2 weeks after a negative initial angiogram. Autopsy search for IAN was not performed because of religious and cultural impediments. The demographic data, age, sex, clinical features, and anatomical location of aneurysm were recorded for analysis. Annual frequencies were calculated and matched with the growth of diagnostic facilities and specialists. The findings were compared to earlier local, regional, and international experiences. The study was not concerned with management or outcome.
Data were entered into spreadsheet and descriptive statistics estimated temporal trends. Frequency tables of variables were generated. Figures and tables were used to illustrate relevant findings.
RESULTS
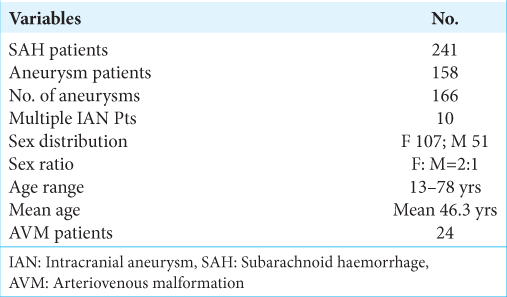
There were 241 SAH patients of which aneurysms were responsible for 158 (65.56%), and AVM in 24 (10%), while for the rest, the cause of SAH could not be confirmed [
There was no aneurysm diagnosed in the first decade of age as the youngest patient was 13 years while the oldest was 78-years-old. The mean age was 48.23 years, the peak incidence was sixth decade, but 37.5% of the patients were 40 years or younger.
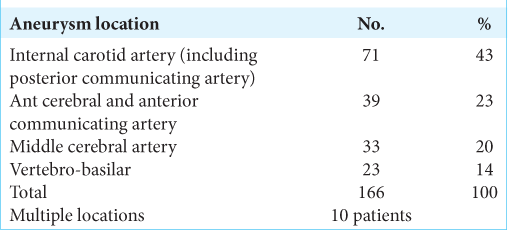
There were 166 aneurysms diagnosed in the 158 patients because of multiple aneurysms in some patients. The internal carotid artery (ICA) was the most frequent location (43%), while others were anterior communicating artery (ACoA) (23%), middle cerebral artery (MCA) (20%), and VertebroBasilar (VBA) (14%). Multiple aneurysms were seen in 10 patients (6.3%) [
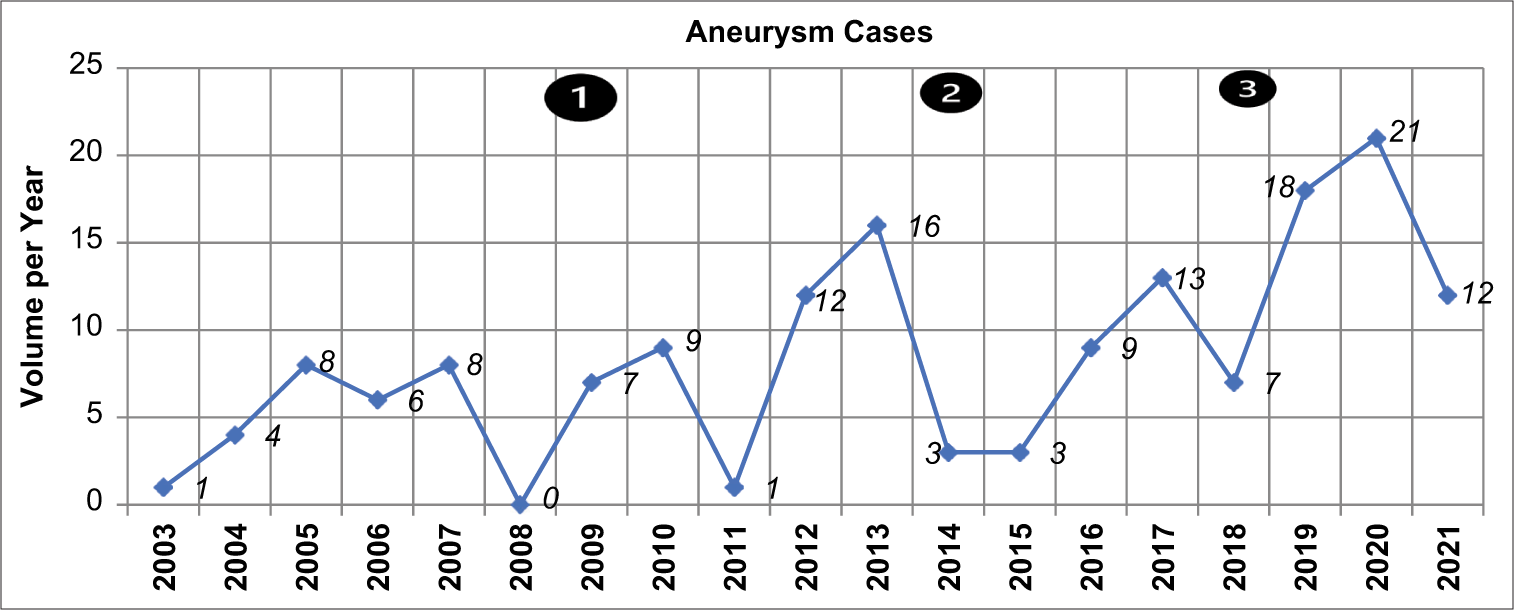
The annual frequency of patients with IAN fluctuated [
The females were more (67.7%), the mean age was 46.3 years, but peak incidence was in the sixth decade.
During the period of the study, 24 cases of AVM were diagnosed giving AVM to IAN ratio of 1:6.6. Two of the patients with AVM also had multiple aneurysms, with one of them harboring four aneurysms.
The clinical presentations of IAN were diverse, but SAH predominated in 65%. Other findings were headaches (92%), 3rd nerve palsy (32), seizures (5), suspected meningitis (2), and sudden blindness (2). Two patients had polycystic kidney disease but there were no cases of Sickle cell disease, HIV, or family history of IAN in the series.
DISCUSSION
Does the study suggest low frequency of Aneurysms in Enugu?
Between 2003 and 2008, the study center depended exclusively on non-digital carotid angiography as there were no facilities for CTA or MRA. The annual frequency of IAN detected ranged from 1 to 8. The introduction of CTA and MRA in 2009 using 8-slice CT and 0.35T MRI did not increase the numbers significantly [
The quality of our diagnostic services improved in 2014 with the installation of a 64-slice CT scanner that provided a high standard CTA, but this did not significantly increase the IAN volumes in the subsequent 3 years as was expected. In 2018, the hospital installed 1.5T MRI that improved MRA quality from the earlier 0.35T machine. Over the 3-year period (2019–2021) there were 51 IAN cases giving an annual frequency of 17 compared to the preceding periods. There was increase in IAN numbers, but considering the large catchment population of the hospital and the fact that it was the referral center for SAH and IAN cases, the results suggest that the frequency of IAN in the sub-region is still low in comparison with international data [
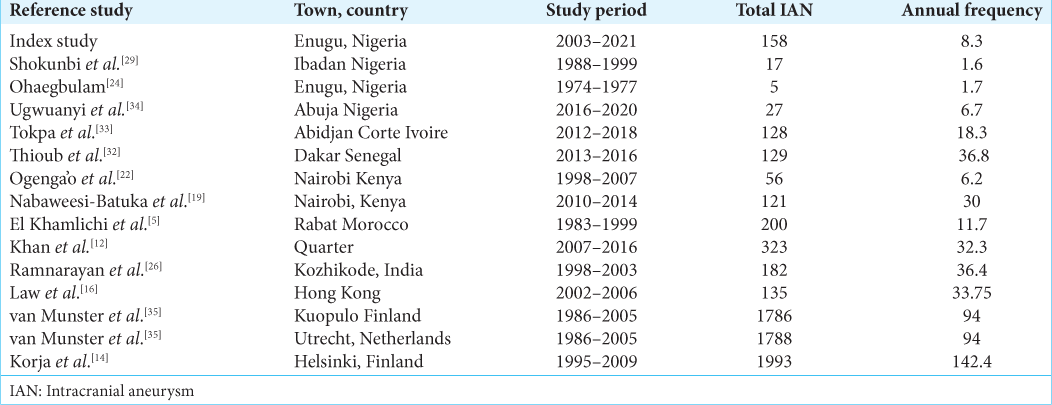
Experiences in other parts of Nigeria and Africa seem to support this view. A study in Abuja, Nigeria,[
A recent scoping review of aneurysmal SAH in Africa revealed the scanty publications on this important subject that dominates neurosurgical literature.[
Interestingly, over a period of 4 years, 82 patients with spontaneous SAH were managed at hospital in Austria, serving 360,000 inhabitants, giving SAH incidence of 5.7/100,000/year. In 70 of the 82 patients (85.4%), cerebral aneurysms were detected by CTA.[
Autopsy studies have provided information on the incidence of IAs although there may be concerns about the accuracy of the results. In an autopsy study of 1000 brains in India, IANs were found in 10 (1%).[
Are IAN characteristics different?
The age distribution in our cohort showed mean age was 48.23 years but the peak incidence was in the 6th decade which is similar to global experience. Females outnumbered males by 2:1 which also is similar to reports from most studies with a few exceptions like a study from Qatar which reported a preponderance of males (68.7%).[
The anatomical distribution of aneurysms showed that ICA which included posterior communicating artery accounted for about 43%, while ACA/ACoA contributed 23% and MCA 20%. This distribution pattern was also recorded in other African studies and in Black Americans unlike Caucasian IAN patients that show dominance of MCA aneurysms,[
Racial and geographical differences in frequency of IANs
Racial differences have been reported between Māori and European populations of New Zealand in aneurysmal SAH. It was found that the incidence per 100,000 of the population for all aneurysms was 14.3 for Europeans but 25.7 for Maoris.[
Other explanations for these differences include genetic predilection and connective tissue disorders. Connective tissue defects may play a significant role in the development of IAN. Multi organ connective tissue disorders may, therefore, indicate a risk of IAN development.[
The major risk factors for IANs are hypertension, tobacco abuse and female sex.[
The uneven sex distribution of IANs suggests possible physiologic or pathologic factors in the intracranial arteries. The female preponderance is usually explained by systemic factors such as hormonal influences and intrinsic wall weakness.[
Despite massive efforts, progress so far has been modest in isolating the genetic determinants for IAN. More detailed epidemiology data might be essential for successful genome-wide association study.
Modern aneurysm management requires heavy investments in financial and human resources to equip specialist centers. Such centers must provide facilities for CTA, MRA, digital subtraction angiography, operating microscopes, vascular C-Arms, endovascular armamentarium, modern intensive care unit, high-dependency unit, and aneurysm clipping tools. The question then arises as how neurosurgical units in countries with low IAN volumes could justify such heavy investments when the healthcare budget is inadequate to even cater for more common diseases. Are the IAN numbers sufficient to produce vascular neurosurgeons? Perhaps the best option might be to identify centers with higher volumes and encourage other centers to refer their cases to those centers. High-volume hospitals have more favorable outcomes than low-volume hospitals. This effect is substantial, even for hospitals conventionally classified as high volume.[
Limitations
Out of pocket payment for healthcare prevents many patients from seeking help. The absence of digital subtraction angiography (DSA) facilities might have led to missing small aneurysms. The COVID-19 pandemic might have prevented some patients from seeking healthcare. Catchment population was based on national census of 2006 and 2016 and for a hospital is not precise.
CONCLUSION
There was an increase in the number of IANs diagnosed in the study center with increasing awareness of the availability of neuroimaging facilities.
Improved diagnostic facilities led to detection of more IANs in the study center. However, the frequency rate was still far less than would be expected for the catchment population of this study, strongly suggesting that improved diagnostic neuroradiology has not debunked rarity of IANs in our subregion.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ammar A, Al-Rajeh S, Ibrahim AW, Chawdhary UM, Awada A. Pattern of subarachnoid haemorrhage in Saudi Arabia. Acta Neurochir (Wien). 1992. 114: 16-9
2. Anim JT. Cerebral macro aneurysms in a Ghanaian adult population. An autopsy studies. Atherosclerosis. 1985. 54: 37-42
3. Bakker MK, Ruigrok YM. Genetics of intracranial aneurysms. Stroke. 2021. 52: 3004-12
4. Connolly ES, Choudhri TF, Mack WJ, Mocco J, Spinks TJ, Slosberg J. Influence of smoking, hypertension, and sex on the phenotypic expression of familial intracranial aneurysms in siblings. Neurosurgery. 2001. 48: 64-9
5. El Khamlichi A, Derra ZS, El Quahabi A, Aghzadi A, Januly , El Azouzi M. Pattern of cerebral aneurysms in Morocco: Review of the concept of their rarity in developing countries: Report of 200 cases. Neurosurgery. 2001. 49: 1224-9
6. Gu YX, Chen XC, Song DL, Leng B, Zhao F. Risk factors for intracranial aneurysm in a Chinese ethnic population. Chin Med J (Engl). 2006. 119: 1359-64
7. Inagawa T, Hirano A. Autopsy study of unruptured incidental intracranial aneurysms. Surg Neurol. 1990. 34: 361-5
8. Inagawa T. Site of ruptured intracranial saccular aneurysms in patients in Izumo City, Japan. Cerebrovasc Dis. 2010. 30: 72-84
9. Iwamoto H, Kiyohara Y, Fujishima M, Kato I, Nakayama K, Sueishi K. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive Autopsy series during a 30-year observation period. The Hisayama study. Stroke. 1999. 30: 1390-5
10. Jalava I, Pyysalo L, Alanen M, Snicker O, Öhman J, Ronkainen A. Regional differences in the incidence of aneurysmal subarachnoid haemorrhage in Finland. Acta Neurochir (Wien). 2017. 159: 1657-62
11. Kapoor K, Kak VK. Incidence of intracranial aneurysms in Northwest Indian population. Neurol India. 2003. 51: 22-6
12. Khan DA, Shaikh DN, Khan DM, Alkubaisi DA, Al Rumaihi DG, Al-Sulaiti DG. Epidemiology of spontaneous subarachnoid hemorrhage in the state of Qatar. Qatar Med J. 2020. 2020: 19
13. King JT. Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997. 7: 659-68
14. Korja M, Kivisaari R, Jahromi BR, Lehto H. Size and location of ruptured intracranial aneurysms: Consecutive series of 1993 hospital-admitted patients. J Neurosurg. 2017. 12: 748-53
15. Kristensen MO. Increased incidence of bleeding intracranial aneurysms in Greenlandic Eskimos. Acta Neurochir (Wien). 1983. 67: 37-43
16. Law HY, Wong GK, Chan DT, Wong L, Poon WS. Meteorological factors and aneurysmal subarachnoid haemorrhage in Hong Kong. Hong Kong Med J. 2009. 15: 85-9
17. Lindekleiv HM, Valen-Sendstad K, Morgan MK, Mardal KA, Faulder K, Magnus JH. Sex differences in intracranial arterial bifurcations. Gend Med. 2010. 7: 149-55
18. Marks PV, Hope JK, Cluroe AD, Furneaux CE. Racial differences between Maori and European New Zealanders in aneurismal subarachnoid haemorrhage. Br J Neurosurg. 1993. 7: 175-81
19. Nabaweesi-Batuka J, Kitunguu PK, Kiboi JG. Pattern of cerebral aneurysms in a Kenyan population as seen at an Urban Hospital. World Neurosurg. 2016. 87: 255-65
20. Naso WB, Rhea AH, Poole A. Management, and outcomes in a low-volume cerebral aneurysm practice. Neurosurgery. 2001. 48: 91-9 discussion 99-100
21. Nguyen TV, Chandrashekar K, Qin Z, Parent AD, Zhang J. Epidemiology of intracranial aneurysms of Mississippi: A 10-year (1997-2007) retrospective study. J Stroke Cerebrovasc Dis. 2009. 18: 374-80
22. Ogeng’o JA, Otieno BO, Kilonzi J, Sinkeet SR, Muthoka JM. Intracranial aneurysms in an African country. Neurol India. 2009. 57: 613-6
23. Ohaegbulam SC, Dujovny M, Ausman JI, Diaz FG, Malik GM. Ethnic distribution of intracranial aneurysms. Acta Neurochir (Wien). 1990. 106: 132-5
24. Ohaegbulam SC. Racial bias in intracranial aneurysms?. Trop Geogr Med. 1978. 30: 305-11
25. Pandey AS, Gemmete JJ, Wilson TJ, Chaudhary N, Thompson BG, Morgenstern LB. High subarachnoid hemorrhage patient volume associated with lower mortality and better outcomes. Neurosurgery. 2015. 77: 462-70 discussion 470
26. Ramnarayan R, Anto D, Alapatt J. Aneurysmal subarachnoid hemorrhage: Geography has a role. Asian J Neurosurg. 2018. 13: 669-73
27. Rautalin I, Lindbohm JV, Kaprio J, Korja M. Substantial within-country variation in the incidence of subarachnoid hemorrhage: A nationwide Finnish study. Neurology. 2021. 97: e52-60
28. Roessler K, Cejna M, Zachenhofer I. Aneurysmatic subarachnoidal haemorrhage: Incidence and location of small ruptured cerebral aneurysms-a retrospective population-based study. Wien Klin Wochenschr. 2011. 123: 444-9
29. Shokunbi MT, Komolafe EO, Badejo OA, Malomo AO, Adeolu AA. Are intracranial aneurysms rare among Nigerian African?. Arch Neurosurg Afr. 2021. 2: 33-8
30. Tetinou F, Kanmounye US, Nitcheu I, Oriaku AJ, Ndajiwo AB, Bankole ND. Cerebral aneurysms in Africa: A scoping review. Interdiscipl Neurosurg. 2021. 26: 101291
31. Thioub M, Mbaye M, Sy EC, Sylla N, Cisse EH, Thiam AB, editors. Surgical Management of Intracranial Aneurysms in Senegal a Prospective study of 65 Patient. p. Available from: https://www.snsa-congress.co.za/wp-content/uploads/2016/10 [Last accessed on 2023 Mar 14]
32. Thioub M, Mbaye M, Thiam AB, Zirhumana C, Sy C, Ndoye N. Microsurgical treatment of ruptured intracranial aneurysms in Sub-Saharan Africa: A series of 102 consecutive cases treated in Senegal. World Neurosurg. 2018. 110: 226-31
33. Tokpa A, Haïdara A, Derou L, Dongo S, Kakou M, Varlet G. Microsurgical management of intracranial aneurysms in Côte d’Ivoire: A series of 128 cases. Open J Mod Neurosurg. 2020. 10: 105-13
34. Ugwuanyi CUAnigbo AANwaribe EEAyogu OMOkpata CISalawu MM. A Review of Aneurysmal Subarachnoid Hemorrhage managed in Abuja North-Central Nigeria. Available from: https://www.crimsonpublishers.com/tnn/fulltext/TNN.000581.php [Last accessed on 2023 Mar 14].
35. van Munster CE, von und zu Freudberg M, Rinkel GJ, Rinne J, Koivisto T, Ronkainen A. Differences in aneurysm and patient characteristics between cohorts of Finnish and Dutch patients with subarachnoid hemorrhage: time trends between 1986 and 2005. Stroke. 2008. 39: 3166-71
36. Vega C, Kwoon JV, Lavine SD. Intracranial aneurysms: Current evidence and clinical practice. Am Fam Physician. 2002. 66: 601-8