- Department of Head and Neck Surgery, Neurosurgery Unit, Garibaldi Hospital, Catania, Italy
- Neurosurgical Clinic, AOUP “Paolo Giaccone,” Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy
- Department of Diagnostic Imaging, Interventional Radiology and Neuroradiology, Garibaldi Hospital, Catania, Italy
- Department of Neurosurgery, Cannizzaro Hospital, Catania, Italy.
Correspondence Address:
Gianluca Scalia, Deaprtment of Head and Neck Surgery, Neurosurgery Unit, Garibaldi Hospital, Catania, Italy.
DOI:10.25259/SNI_66_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gianluca Scalia1, Roberta Costanzo2, Manikon Poullay Silven2, Domenico Gerardo Iacopino2, Giovanni Federico Nicoletti1, Gianluca Galvano3, Giuseppe Emmanuele Umana4. Case of incidental thoracic spinal dumbbell hemorrhagic arachnoid cyst and tentorial metastasis from breast carcinoma. 10-Feb-2023;14:50
How to cite this URL: Gianluca Scalia1, Roberta Costanzo2, Manikon Poullay Silven2, Domenico Gerardo Iacopino2, Giovanni Federico Nicoletti1, Gianluca Galvano3, Giuseppe Emmanuele Umana4. Case of incidental thoracic spinal dumbbell hemorrhagic arachnoid cyst and tentorial metastasis from breast carcinoma. 10-Feb-2023;14:50. Available from: https://surgicalneurologyint.com/surgicalint-articles/12148/
Abstract
Background: Spinal arachnoid cysts (SACs) in adults are typically acquired dural defects following trauma, inflammation, or infection. Brain metastases from breast cancer account for 5–12% of all CNS metastases and are mostly leptomeningeal. Here, the authors reported a 50-year-old female treated for a tentorial metastasis from breast carcinoma that underwent chemotherapy and radiotherapy. Three months later, she presented with a thoracic spinal extradural dumbbell hemorrhagic arachnoid cyst.
Case Description: A 50-year-old female underwent a left retrosigmoid suboccipital craniectomy for microsurgical removal of a tentorial metastasis attributed to poorly differentiated breast carcinoma (i.e., comedonic pattern). The patient subsequently underwent both chemotherapy and radiotherapy for accompanying bony metastases. Three months later, she experienced the onset of severe posterior thoracic pain. When the thoracic magnetic resonance imaging revealed a hyperintense “dumbbell” extradural T10–T11 lesion, she underwent a T10–T11 laminectomy for marsupialization and excision of the hemorrhagic lesion. The histological examination revealed blood and arachnoid tissue within a benign SAC, without accompanying tumor. Her postoperative course was uneventful, and she was discharged on postoperative day 3.
Conclusion: A 50-year-old female underwent a left retrosigmoid suboccipital craniectomy for removal of a tentorial metastasis from breast carcinoma, followed by radiation/chemotherapy. Three months later, she hemorrhaged into an MR-documented T10–T11 dumbell extradural SAC that was successfully treated with laminectomy, marsupialization, and excision.
Keywords: Arachnoid cyst, Dumbbell, Hemorrhage, Spine, Tentorium
INTRODUCTION
Spinal arachnoid cysts (SACs) in adults typically present with back pain and/or signs of myelopathy. They are physiologically attributed to acquired dural defects following trauma, inflammation, or infection.[
CASE PRESENTATION
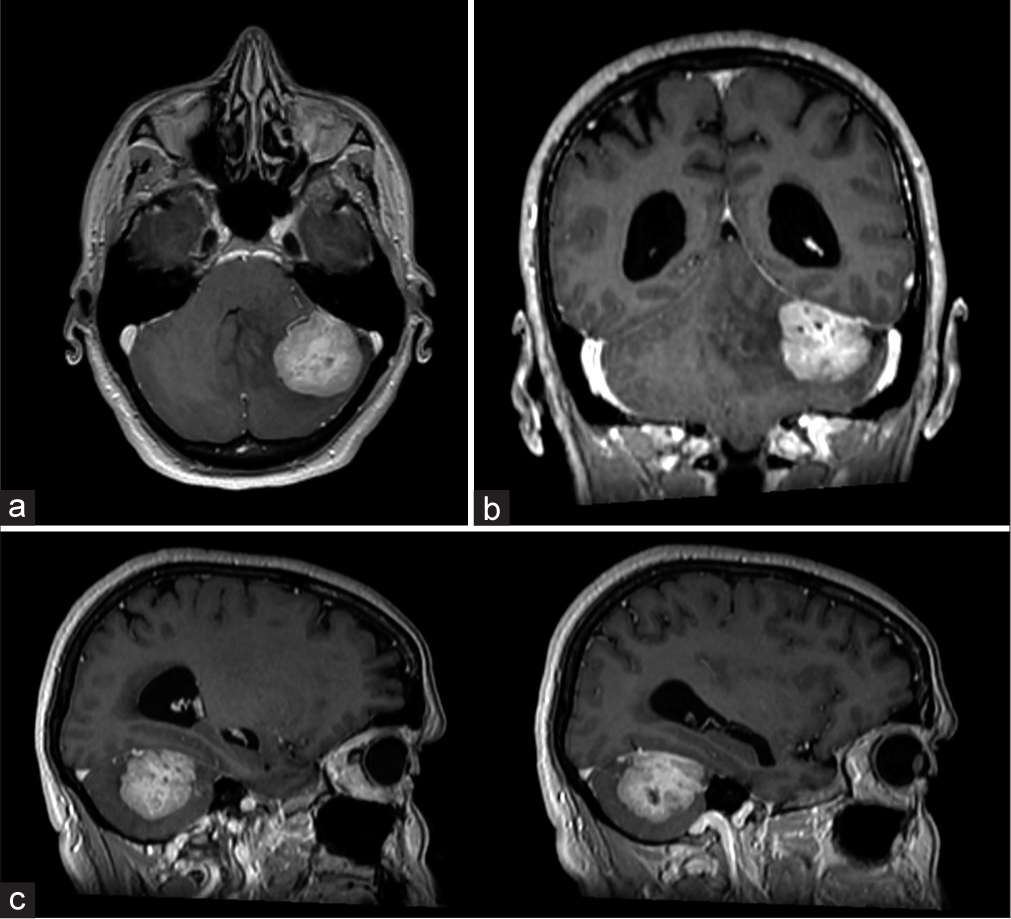
Seven years earlier, she underwent a left inferior quadrantectomy and hormonal/radiotherapy for a Grade II breast-ductal carcinoma in situ. She had undergone (Eastern Cooperative Oncology Group [ECOG] performance status 0) chemotherapy (cyclin inhibitor) and radiotherapy for bony metastases (ten therapy sessions with a daily fractionation of 3 Gy and a total of 30 Gy). This 50-year-old female now presented with worsening headache and nausea but neurologically intact, attributed to a computed tomography (CT) documented large left cerebellar slightly hyperdense lesion (i.e., 43 × 40 mm), with a wide tentorial dural base. The contrast brain magnetic resonance imaging (MRI) showed a large left cerebellar lesion (i.e. 45 mm × 40 mm × 24 mm) that inhomogeneously enhanced with an accompaning tentorial“dural tail” highly consistent with a meningioma [
Figure 1:
Axial (a), coronal (b), and sagittal (c) T1-weighted magnetic resonance imaging with gadolinium images showing a left cerebellar lesion with a maximum diameter of 43 × 40 mm, with inhomogeneous enhancement, tentorial attachment and “dural tail” sign, highly suggestive for left tentorial meningioma.
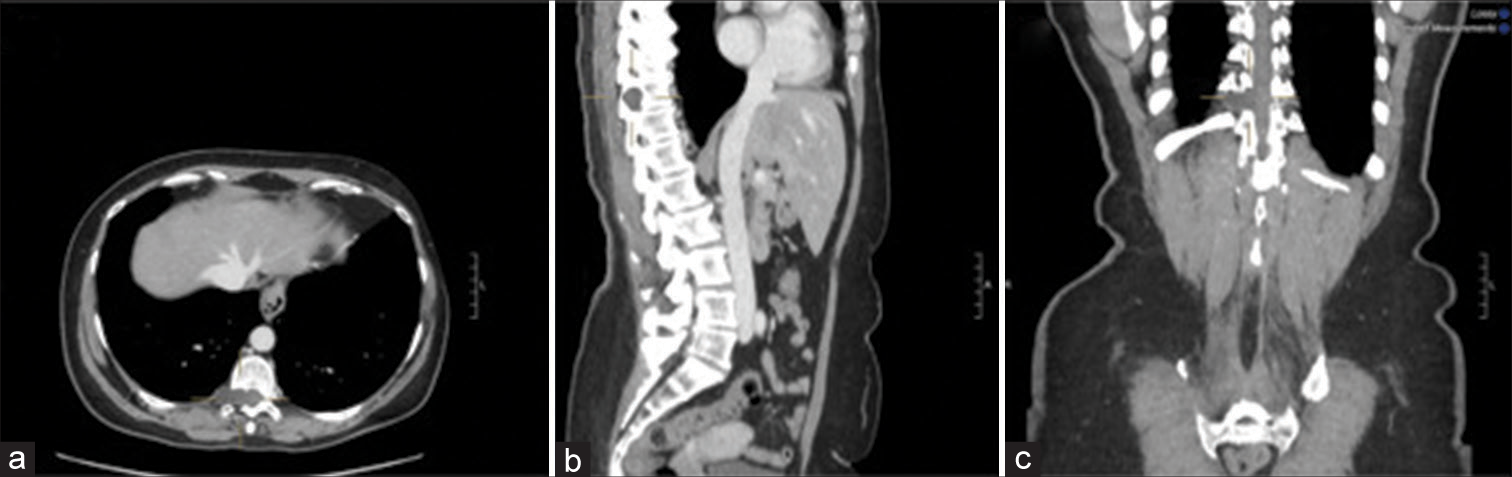
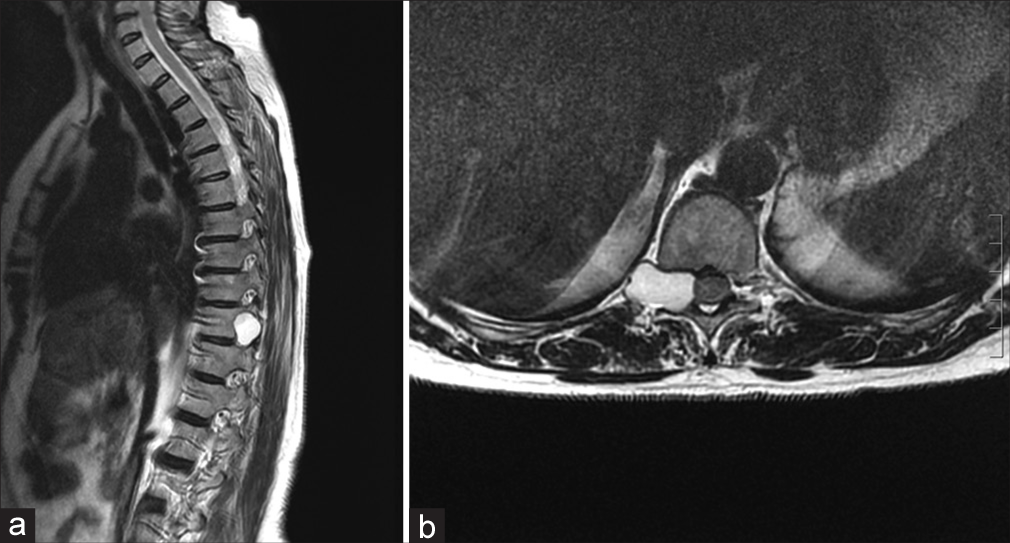
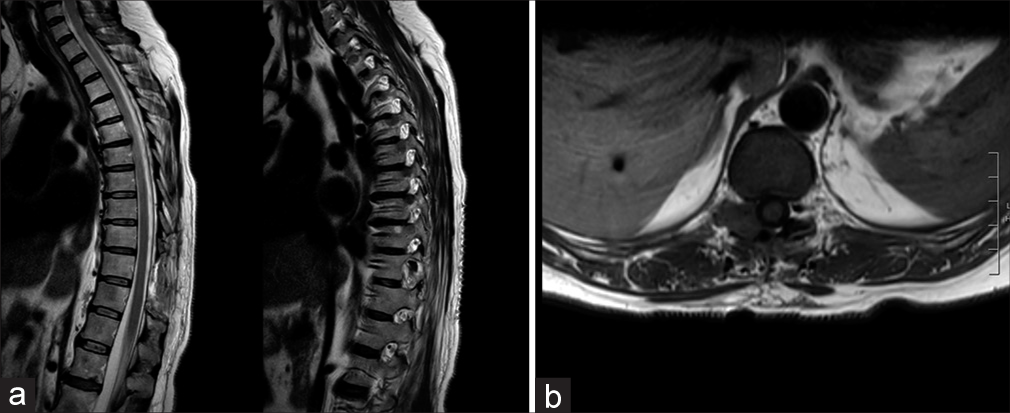
Three months later, she presented with intense posterior thoracic pain (VAS score 7/10) and paresthesia, but neurologically intact. The whole body CT examination documented a hypodense “dumbbell” T10–T11 lesion (i.e., 26 mm) involving the right vertebral pedicle/T10 lamina [
SAC surgery and adjunctive therapies
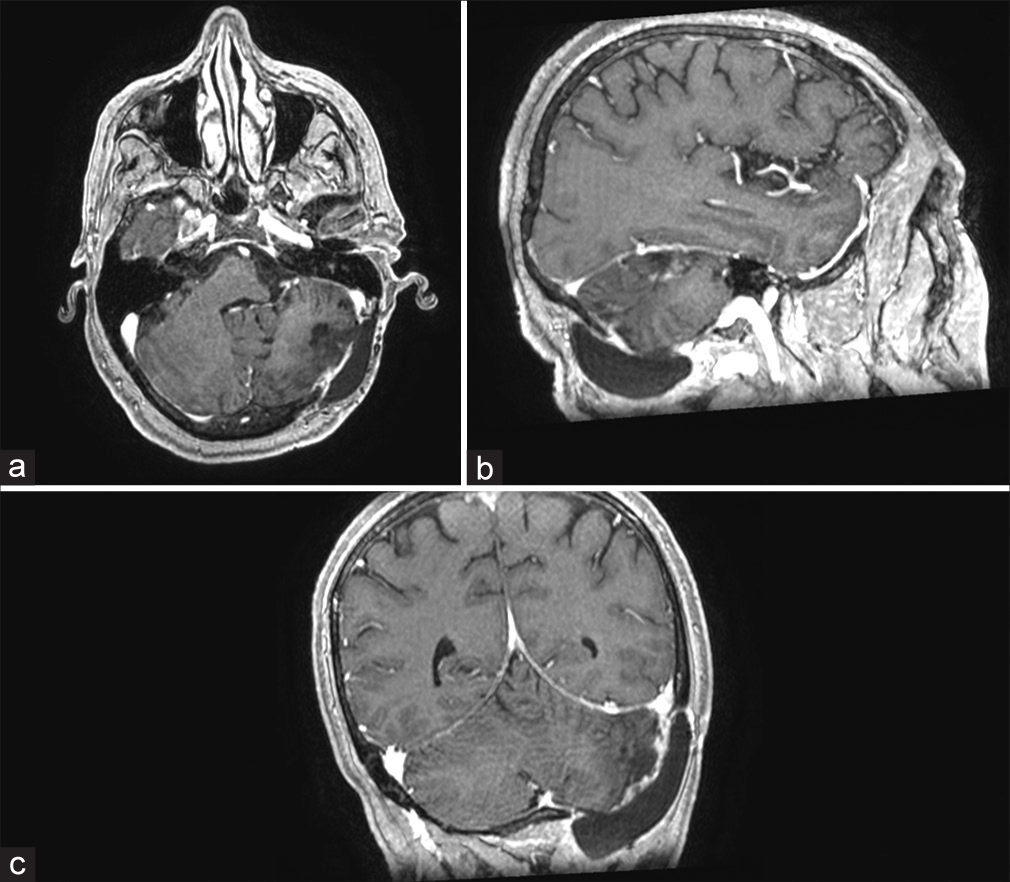
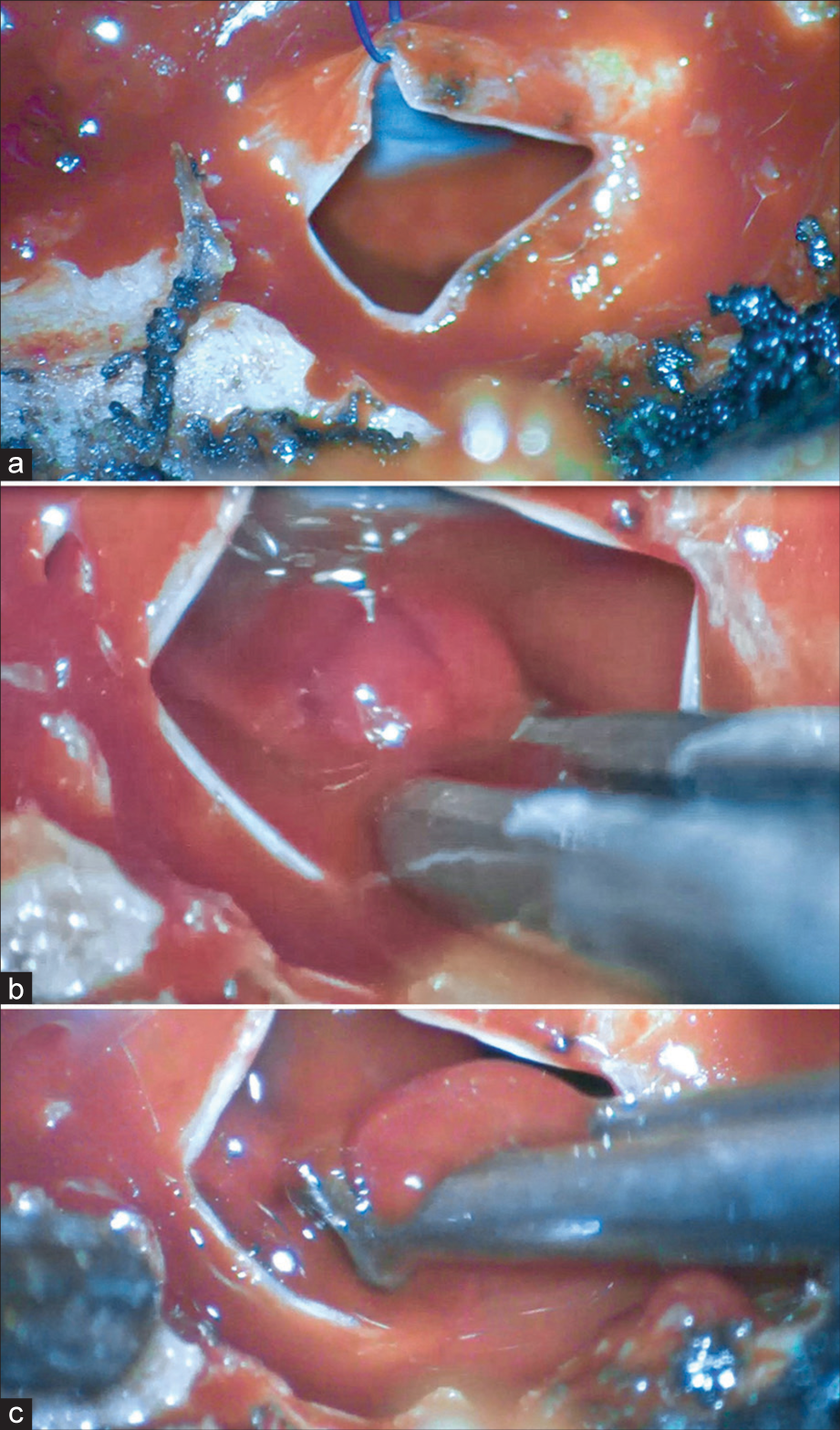
She underwent a T10–T11 laminectomy that revealed an extradural lesion with a capsule indistinguishable from the dura. The capsule was incised, marsupialized, and removed, along with resection of the hemorrhage [
Histology and immunohistochemistry of the breast metastasis
The histological examination documented a tentorial metastasis from poorly differentiated carcinoma. Within the nodular, there were areas of central necrosis (comedonic pattern). Immunohistochemistry was positive for GATA-Binding protein 3 (GATA-3), Epithelial membrane antigen (EMA) and estrogen receptors, negativity for progesterone receptors, chromogranin, human epidermal growth factor receptor 2, synaptophysin, Thyroid Transcription Factor 1 (TTF-1), Cytokeratin 7 (CK7), p40, and a Ki67 of 70%. The morphological and immunohistochemical profile indicated the mammary origin of the neoplasm.
Histology of SAC
The histological examination of the spinal extradural lesion showed blood and arachnoid within sclerotic tissue, consistent with a SAC.
DISCUSSION
Etiology of SACs
SACs are benign lesions commonly found in pediatric patients with accompanying congenital disorders. SACs in adults are typically idiophatic , or rarely related to trauma, infection, inflammation, or iatrogenic.[
Classification and treatment
According to Nabors classification, SAC can be divided in extradural cysts without nerve root involvement (type I) (i.e., subclassified in IA “extradural arachnoid cyst” and IB “sacral meningocele”); II extradural extramedullary cysts with spinal nerve fibers involvement (i.e., “Tarlov’s perineural cyst” or “spinal nerve root diverticulum”); III spinal intradural cysts (i.e., “intradural arachnoid cyst”).[
Here, the unusual onset of hemorrhage into this T10/T11 lesion was attributed to the prior radiation therapy. The main surgical treatment is the surgical decompression followed by resection, fenestration/ marsupialization of the cyst, and/or the placement of a cystoperitoneal/subarachnoid shunt.
Tentorial breast metastasis
Metastases from breast cancer, the most common tumors causing intracranial dural metastases, spread through the leptomeningeas. Nevertheless, they can also be found attached to dural sites.[
CONCLUSION
This case involved a patient with a cranial metastasis from breast cancer (i.e., diagnosed 7 years earlier as an in situ lesion) who also later incidentally presented with a spontaneous hemorrhage into a T10–T11 dumbbell extradural SAC that was successfully excised.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ehsan H, Imtiaz H, Sana MK, Sheikh MM, Wahab A. Relapsed breast cancer complicated by isolated brain metastasis. Clin Case Rep. 2020. 9: 887-90
2. Fam MD, Woodroffe RW, Helland L, Noeller J, Dahdaleh NS, Menezes AH. Spinal arachnoid cysts in adults: Diagnosis and management. A single-center experience. J Neurosurg Spine. 2018. 29: 711-9
3. Hong JC, Kim MS, Chang CH, Kim SH. Arachnoid cyst with spontaneous intracystic hemorrhage and chronic subdural hematoma. J Korean Neurosurg Soc. 2008. 43: 54-6
4. Iaconetta G, Esposito M, Maiuri F, Cappabianca P. Arachnoid cyst with intracystic haemorrhage and subdural haematoma: Case report and literature review. Neurol Sci. 2006. 26: 451-5
5. Kadono Y, Yuguchi T, Ohnishi Y, Iwatsuki K, Yoshimine T. A symptomatic spinal extradural arachnoid cyst with lumbar disc herniation. Case Rep Orthop. 2015. 2015: 250710
6. Marrone S, Kharbat AF, Palmisciano P, Umana GE, Haider AS, Iacopino DG. Thoracic spinal extradural arachnoid cyst: A case report and literature review. Surg Neurol Int. 2022. 13: 55
7. Mastantuoni C, Pizzuti V, Ricciardi F, D’Elia A, Leonetti S, Colonnese C. Cervical spine arachnoid cyst complicated by spontaneous intracystic hemorrhage: Case report and review of the literature. Surg Neurol Int. 2022. 13: 427
8. Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg. 1988. 68: 366-77
9. Nayak L, Abrey LE, Iwamoto FM. Intracranial dural metastases. Cancer. 2009. 115: 1947-53
10. Schmutzer M, Tonn JC, Zausinger S. Spinal intradural extramedullary arachnoid cysts in adults-operative therapy and clinical outcome. Acta Neurochir (Wien). 2020. 162: 691-702