- Department of Neurosurgery, Fukuoka University, Fukuoka, Japan.
Correspondence Address:
Takashi Morishita, Department of Neurosurgery, Fukuoka University, Fukuoka, Japan.
DOI:10.25259/SNI_618_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kazunori Oda, Takashi Morishita, Hideaki Tanaka, Hiromasa Kobayashi, Hiroshi Abe. Case report: Radiofrequency thalamotomy as palliative care for Holmes tremor in a patient with terminal cancer and cardiac pacemaker. 21-Oct-2022;13:484
How to cite this URL: Kazunori Oda, Takashi Morishita, Hideaki Tanaka, Hiromasa Kobayashi, Hiroshi Abe. Case report: Radiofrequency thalamotomy as palliative care for Holmes tremor in a patient with terminal cancer and cardiac pacemaker. 21-Oct-2022;13:484. Available from: https://surgicalneurologyint.com/surgicalint-articles/11941/
Abstract
Background: Herein, we present a case report of a patient with Holmes tremor due to thalamic infarction with end-stage pancreatic cancer who underwent successful computed tomography (CT)-guided ventralis intermedius nucleus (Vim) thalamotomy as palliative care.
Case Description: A 78-year-old man with gradually worsening involuntary movements on the left side of his body 2 years after a right thalamic infarction was referred to our institute. He had a history of chronic atrial fibrillation for which he was implanted with a cardiac pacemaker not compatible with magnetic resonance imaging. He also received adjuvant therapy for pancreatic cancer. As the involuntary movements interfered with his daily life, the patient elected for neurosurgical treatment despite having terminal cancer. Although the prognosis for pancreatic cancer was considered to be more than 6 months at the time of surgery, we performed CT-guided Vim thalamotomy under local anesthesia without pulse generator implantation considering the patient’s general condition. The involuntary movements of the left side of the body reduced following surgery, thus improving his quality of life (QOL). However, 6 months after thalamotomy, the patient died of pancreatic cancer.
Conclusion: Thalamotomy significantly reduced the involuntary movements immediately after the procedure. Therefore, thalamotomy can be performed under local anesthesia without the use of any device and may contribute to the improvement of QOL in terminal patients.
Keywords: Functional neurosurgery, Holmes tremor, Palliative care, Radiofrequency, Thalamotomy
INTRODUCTION
Holmes tremor (HT) is generally associated with cerebellum lesions, the midbrain, or the thalamus.[
CASE REPORT
A 78-year-old man with a history of a pacemaker implantation for chronic atrial fibrillation not compatible with MRI and in the process of chemotherapy for pancreatic cancer visited our department complaining of severe left-sided tremors. He had a right thalamic infarction 2 years before his visit and developed involuntary movements and dysesthesia on the left side of his body a few months later. As involuntary movements gradually aggravated and started interfering with his daily life, the patient was referred to our department for treatment. At the patient’s request, surgical treatment for symptom relief was considered. Although the prognosis for pancreatic cancer was considered to be more than 6 months at the time of surgery, we performed CT-guided Vim thalamotomy under local anesthesia without pulse generator implantation considering the patient’s general condition.
Stereotactic CT scan with contrast was preoperatively used to identify the target and plan a safe trajectory to avoid blood vessels on the brain surface and around the ventricles. We targeted the area near the middle frontal gyrus that is at the lower end of the convergence range. The boundary between the posterior internal capsule and thalamus was aimed and targets were determined based on the site of infarction within the thalamus.
The preoperative stereotactic targeting (tip of the electrode) coordinates relative to the mid-commissural point and trajectory angles were as follows: 10.5 mm to the right, 5.5 mm posterior, 1.0 mm superior, anterior commissure-posterior commissure angle of 67.3°, and coronal plane angle of 28.5° to the right. Since the CT scan has difficulty showing a high-resolution structural image, we used the previous thalamic infarction as a landmark of the ventralis caudalis (Vc) nucleus and the border between the thalamus and the internal capsule. The tentative trajectory was aimed at the Vc/ Vim border.[
On the morning of surgery, a Leksell G frame (Elekta, Stockholm, Sweden) was attached to the patient’s head under local anesthesia, and subsequently stereotactic CT scan was performed. After a 4-cm straight skin incision, a burr hole was fashioned along the trajectory. Following dural opening, a thermocoagulation electrode was inserted to the target. Subsequently, macrostimulation was performed using 1-mm diameter, 4-mm length electrodes at 100 μs, 133 Hz up to 5 mA to confirm that no adverse effects occurred followed by radiofrequency coagulation at 70°C for 60 s. Another radiofrequency lesion was made along the trajectory, 2 mm anterior and 1 mm medial to the first lesion aiming at the border between the Vim and the ventralis oralis nucleus.
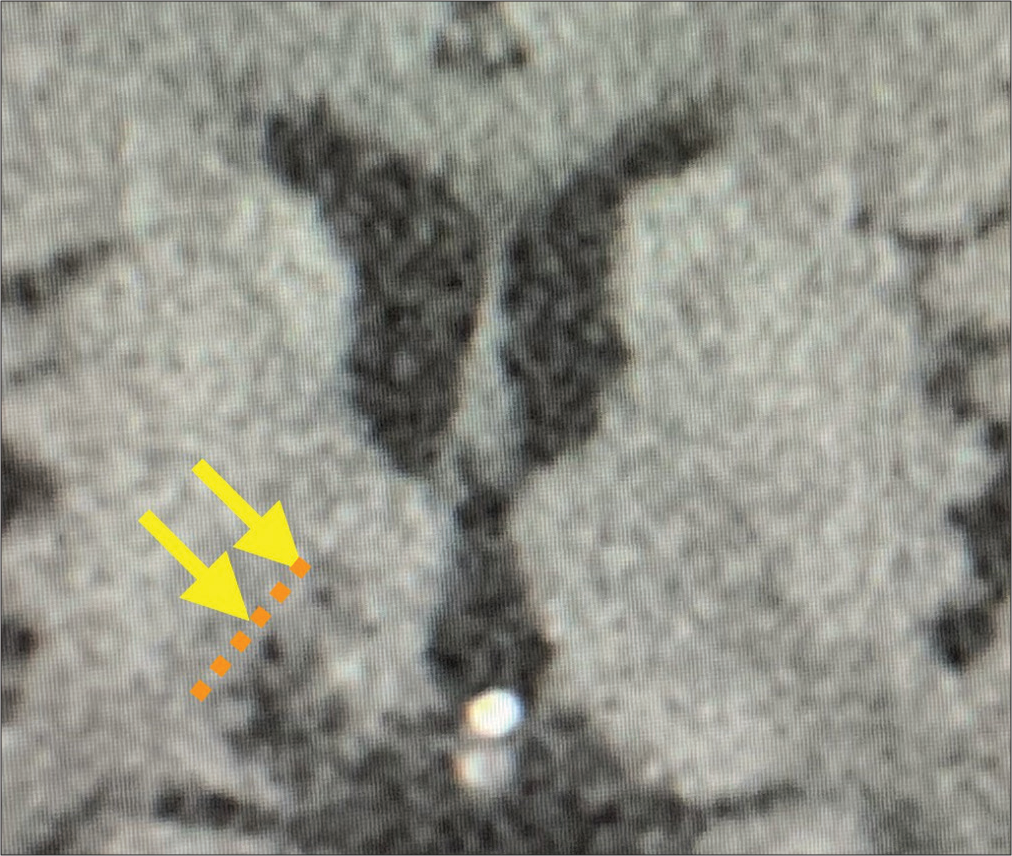
Following the surgery, the involuntary movements of the left side of the body reduced although ataxia remained. Postoperative CT showed an appropriate lesion in the right thalamus [
DISCUSSION
Although long-term follow-up was not performed, stereotactic thalamotomy was considered to be successful in the reported patient. Based on the previous papers, it is suggested that a larger lesion of the thalamotomy was more effective than a smaller lesion.[
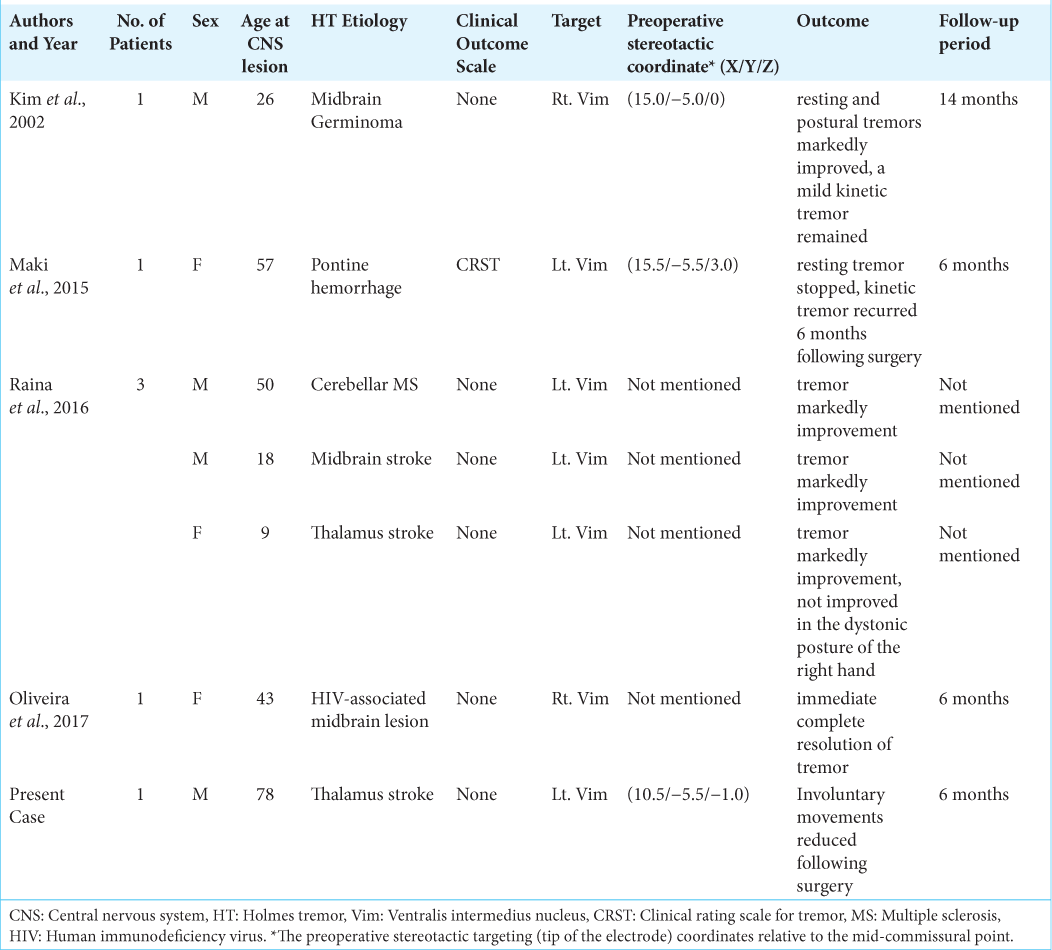
We conducted a literature search for all previous case reports and clinical research published in English on HT treated with thalamotomy since the Consensus Statement of the Movement Disorder Society coined the current definition of HT in 1998. PubMed, MEDLINE, and other databases such as OvidSP were searched using the following keywords: “radiofrequency thalamotomy” and “HT,” “rubral or midbrain tremor,” or “cerebellar outflow tremor.” Four reports and six cases of HT treated with radiofrequency thalamotomy have been reported [
As the previous literature indicated, lesion therapy is considered to be a reasonable option in terminal cases. Keep et al. reported thalamotomy with gamma-knife surgery for the palliative management of thalamic pain.[
CONCLUSION
We report a case of Vim thalamotomy for thalamic infarction-induced HT that showed significant improvement in tremors immediately after the procedure. According to the literature, the Vim thalamotomy outcome is acceptably favorable. The procedure can be safely performed under local anesthesia without the use of any device; hence, thalamotomy may contribute to the improvement of QOL in terminal patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was partly supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C) (Grant number: 18K08956).
Conflicts of interest
There are no conflicts of interest.
Commentary
The authors of this report went an extra mile to help the patient with disabling post-stroke tremor. Although there were many possible reasons to deny surgery (the advanced age, terminal disease, inability to have MRI), they decided to give the patient a chance for living the rest of his life (ended up being 6 months) with significant improvement in symptoms through a very elegant intervention performed under local anesthesia. The approach and the modality they used has been described in the past, but since the relative rarity of this condition precludes one from putting together a large clinical series, it would be worthwhile to review this case as it describes something that was probably the only reasonable way to manage this particular individual case. Instead of finding many excuses not to help this patient, the authors used a time-tested intervention (radiofrequency thermothalamotomy for tremor by far precedes currently used deep brain stimulation and MR-guided focused ultrasound thalamotomy) that is remarkably safe if performed by an experienced team.
Konstantin Slavin, Chicago, Illinois University of Illinois at Chicago; Chicago, IL, USA kslavin@uic.edu
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
Consent to publish statement: Additional informed consent was obtained from the patient for whom identifying information was included in this study.
References
1. Akkus DE, Diramali AB. Postischemic delayed Holmes’ tremor responding to low-dose cabergoline. Mov Disord. 2006. 21: 733-4
2. Bargiotas P, Nguyen TA, Bracht T, Mürset M, Nowacki A, Debove I. Long-term outcome and neuroimaging of deep brain stimulation in Holmes tremor: A case series. Neuromodulation. 2021. 24: 392-9
3. Dallapiazza RF, Lee DJ, De Vloo P, Fomenko A, Hamani C, Hodaie M. Outcomes from stereotactic surgery for essential tremor. J Neurol Neurosurg Psychiatry. 2019. 90: 474-82
4. Deuschl G, Bain P, Brin M. Consensus statement of the movement disorder society on tremor. Mov Disord. 1998. 13: 2-23
5. Haddad AR, Hayley J, Mostofi A, Brown M, Pereira E. Stereotactic radiofrequency thalamotomy for cancer pain: A systematic review. World Neurosurg. 2021. 151: 225-34
6. Hirai T, Miyazaki M, Nakajima H, Shibasaki T, Ohye C. The correlation between tremor characteristics and the predicted volume of effective lesions in stereotaxic nucleus ventralis intermedius thalamotomy. Brain. 1983. 106: 1001-18
7. Holmes G. On certain tremors in organic cerebral lesions. Brain. 1904. 27: 327-75
8. Kim MC, Son BC, Miyagi Y, Kang JK. Vim thalamotomy for Holmes’ tremor secondary to midbrain tumour. J Neurol Neurosurg Psychiatry. 2002. 73: 453-5
9. Keep MF, Mastrofrancesco L, Craig AD, Ashby LS. Gamma knife surgery targeting the centromedian nucleus of the thalamus for the palliative management of thalamic pain: Durable response in stroke-induced thalamic pain syndrome. J Neurosurg. 2006. 105: 222-8
10. Liou LM, Shih PY. Successful treatment of rubral tremor by high-dose trihexyphenidyl: A case report. Kaohsiung J Med Sci. 2006. 22: 149-53
11. Maki F, Sato S, Watanabe K, Yanagisawa T, Hagiwara Y, Shimizu T. Vim thalamotomy in a patient with Holmes’ tremor and palatal tremor-pathophysiological considerations. BMC Neurol. 2015. 15: 26
12. Morishita T, Higuchi MA, Kobayashi H, Abe H, Higashi T, Inoue T. A retrospective evaluation of thalamic targeting for tremor deep brain stimulation using high-resolution anatomical imaging with supplementary fiber tractography. J Neurol Sci. 2019. 398: 148-56
13. Morishita T, Tsuboi Y, Higuchi MA, Inoue T. Is one large target better than two?. J Neurosurg. 2015. 123: 1349-50
14. Niranjan A, Jawahar A, Kondziolka D, Lunsford LD. A comparison of surgical approaches for the management of tremor: Radiofrequency thalamotomy, gamma knife thalamotomy and thalamic stimulation. Stereotact Funct Neurosurg. 1999. 72: 178-84
15. Oliveira JO, Cecilio SA, Oliveira M, Takahashi LR, Galassi AR, Holanda VM. VIM thalamotomy in the treatment of Holmes’ tremor secondary to HIV-associated midbrain lesion: A case report. J Neurosurg Sci. 2017. 61: 544-6
16. Raina GB, Cersosimo MG, Folgar SS, Giugni JC, Calandra C, Paviolo JP. Holmes tremor: Clinical description, lesion localization, and treatment in a series of 29 cases. Neurology. 2016. 86: 931-8
17. Tripathi M, Mehta S, Singla R, Ahuja CK, Tandalya N, Tuleasca C. Vim stereotactic radiosurgical thalamotomy for drug-resistant idiopathic Holmes tremor: A case report. Acta Neurochi (Wien). 2021. 163: 1867-1
18. Wang KL, Wong JK, Eisinger RS, Carbunaru S, Smith C, Hu W. Therapeutic advances in the treatment of Holmes tremor: Systematic review. Neuromodulation. 2022. 25: 796-803