- Department of Neurological Surgery, Keck School of Medicine of University of Southern California, Los Angeles, California, United States
- Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California, United States
- Department of Preventive Medicine, Keck School of Medicine of University of Southern California, Los Angeles, California, United States.
Correspondence Address:
Kristie Qwan-Ting Liu, Department of Neurological Surgery, Keck School of Medicine of USC, Los Angeles, California, United States.
DOI:10.25259/SNI_440_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kristie Qwan-Ting Liu1, Jonathan Dallas1, Talia A. Wenger1, Hunter Richards2, Li Ding3, Frances Elaine Chow1, Gabriel Zada1, William J. Mack1, Frank J. Attenello1. Coronavirus disease-19 is associated with decreased treatment access and worsened outcomes in malignant brain tumor patients. 18-Aug-2023;14:292
How to cite this URL: Kristie Qwan-Ting Liu1, Jonathan Dallas1, Talia A. Wenger1, Hunter Richards2, Li Ding3, Frances Elaine Chow1, Gabriel Zada1, William J. Mack1, Frank J. Attenello1. Coronavirus disease-19 is associated with decreased treatment access and worsened outcomes in malignant brain tumor patients. 18-Aug-2023;14:292. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12506
Abstract
Background: The global coronavirus disease-19 (COVID-19) pandemic has resulted in procedural delays around the world; however, timely and aggressive surgical resection for malignant brain tumor patients is essential for outcome optimization. To investigate the association between COVID-19 and outcomes of these patients, we queried the 2020 National Inpatient Sample (NIS) for differences in rates of surgical resection, time to surgery, mortality, and discharge disposition between patients with and without confirmed COVID-19 infection.
Methods: Patient data were taken from the NIS from April 2020 to December 2020. COVID-19 diagnosis was determined with the International Classification of Diseases, Tenth Revision, Clinical Modification code U07.1.
Results: A total of 30,671 malignant brain tumor patients met inclusion criteria and 738 (2.4%) patients had a confirmed COVID-19 diagnosis. COVID-19-positive patients had lower likelihood of receiving surgery (Odds ratio [OR] 0.43, 95% confidence interval [CI] 0.29–0.63, P P P P = 0.17).
Conclusion: COVID-19 infection was associated with worse patient outcome in malignant brain tumor patients, including decreased likelihood of receiving surgery, increased likelihood of mortality, and increased likelihood of non-routine discharge. Our study highlights the need to balance the risks and benefits of delaying surgery for malignant brain tumor patients with COVID-19. Although the COVID-19 pandemic is no longer a public health emergency, understanding the pandemic’s impact on outcome provides important insight in effective triage for these patients in the situations where resources are limited.
Keywords: Brain tumor, Coronavirus, Coronavirus disease-19, Malignant, National inpatient sample
INTRODUCTION
SARS-CoV-2, the virus that causes coronavirus disease-19 (COVID-19), was first identified in December 2019 and by March 2020, it was declared a global pandemic.[
Approximately 25,000 cases of malignant central nervous system cancers occur annually in the United States, representing only 1.4% of total cancer incidence.[
For patients with malignant brain tumors who are COVID-19-positive, the risks and benefits of urgent resection must be weighed. The American Society of Anesthesiologists guidelines recommend waiting 7 weeks after COVID-19 diagnosis for surgeries when possible to minimize postoperative risks.[
In this study, we utilize the National Inpatient Sample (NIS) database to investigate the national rates of surgical resection, mortality, and unfavorable discharge in COVID-19-positive patients with malignant brain tumors. To the best of our knowledge, our study is the first to use national data to analyze the association of a COVID-19 diagnosis with the outcomes of likelihood of receiving surgery, mortality, and discharge disposition in malignant brain tumor patients.
MATERIALS AND METHODS
Data source
The Healthcare Cost and Utilization Project (HCUP), an initiative by the Agency for Healthcare Research and Quality (AHRQ), releases the NIS annually. This comprehensive database is the most extensive publicly available inpatient health-care database in the United States and covers over 97% of the national population. The NIS comprises approximately 7 million hospital stays each year without weighting, and about 35 million hospitalizations with weighting.[
Study population
This study is a retrospective database analysis of NIS data from April 2020 to December 2020, which are the first dates to include the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code (U07.1) for COVID-19 infection. Patients diagnosed with malignant neoplasms of the brain were included in the study. Patients with benign brain tumors were excluded from the cohort. A full list of ICD-10 codes used in inclusion/exclusion criteria can be found in Supplementary Table S1. Patients who were under 18 years old, missing in-hospital survival data, missing length of stay data, or missing discharge disposition were also excluded from the study.
Study variables
To conduct univariate and multivariate analyses, data were extracted from the NIS database for patient, hospital, and clinical information. COVID-19 was the primary exposure variable (ICD-10 code U07.1). Patient characteristics analyzed included age, sex, race, insurance status/payer, All
Patient Refined Diagnosis Related Groups (APR-DRG) Risk of, APR-DRG Severity of illness, Elixhauser comorbidity index (ECI), patient residential location, and income. Hospital characteristics include bed size (i.e., number of hospital beds), control/ownership of hospital, census division of the hospital, and census region of hospital. Outcome variables included surgical resection (on a binary yes/no scale), in-hospital mortality, discharge disposition (unfavorable vs. favorable), and transfer status. A full breakdown of each variable used in the study as shown in
The above covariates were utilized to evaluate the effect of COVID-19 on the primary outcomes of (1) rate of surgical resection, (2) in-hospital mortality, and (3) discharge disposition. APR-DRG risk of mortality and disease severity was assigned using software developed by 3M Health Information Systems, and the ECI was grouped into four categories (0, 1, 2, and ≥3 factors) based on an ICD-10 coding algorithm.
Statistical analysis
Descriptive analyses of patient demographics and clinical characteristics were stratified by COVID-19 status using mean (standard deviation) and t-test for continuous variables and frequency (percentage) and χ2 tests for categorical variables. Two types of multivariable regression were then utilized to analyze outcomes: (1) logistic regression for surgical resection and in-hospital mortality and (2) log-binomial regression for discharge disposition and transfer-in status. Generalized estimation equations were used in all models to account for hospital clustering. The logistic model assumptions were checked using the Hosmer-Lemeshow goodness-of-fit test. All of the covariates mentioned earlier were included in the model for adjustment. Age was recategorized as it violated the linearity assumption. Only complete cases were used for regressions without imputation since missing data affected <4% of records. P < 0.05 was considered statistically significant. The analysis was performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Patient demographics
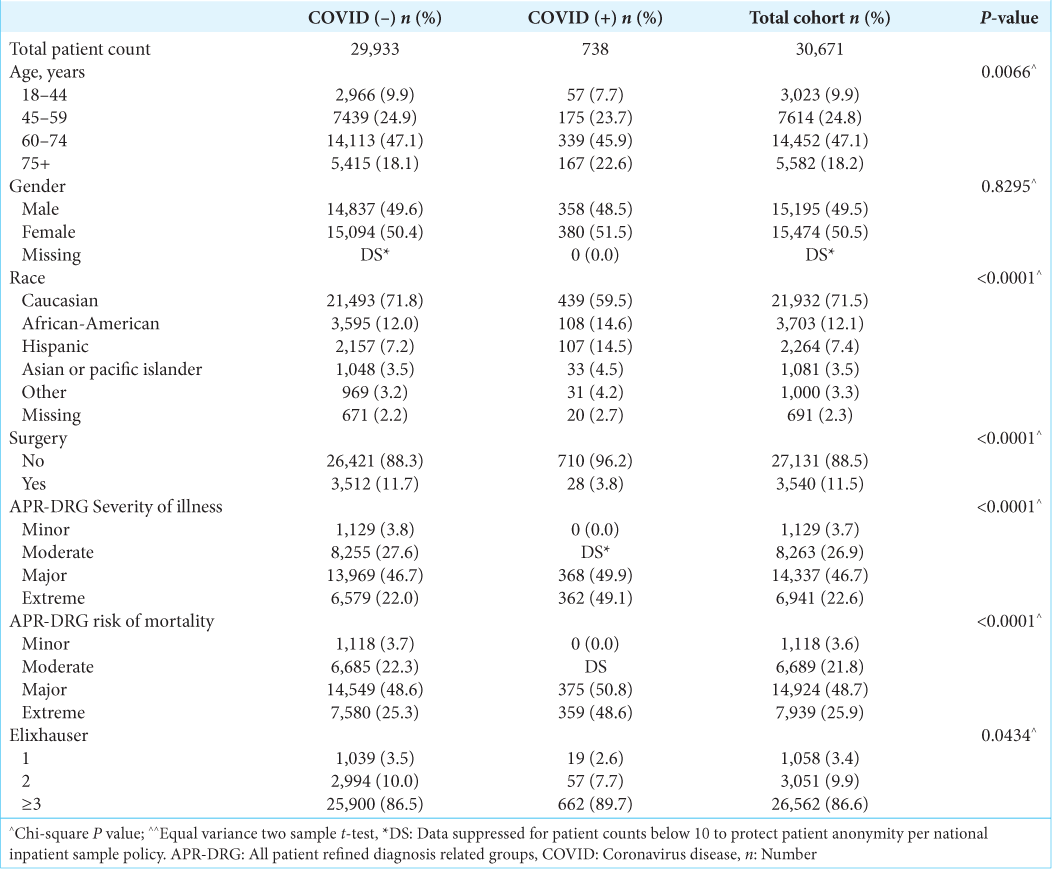
A total of 30,671 patients met inclusion and exclusion criteria. Within this group, 738 (2.4%) patients had a COVID-19 diagnosis. A flowchart outlining the inclusion and exclusion process is shown in
Surgical resection cohort
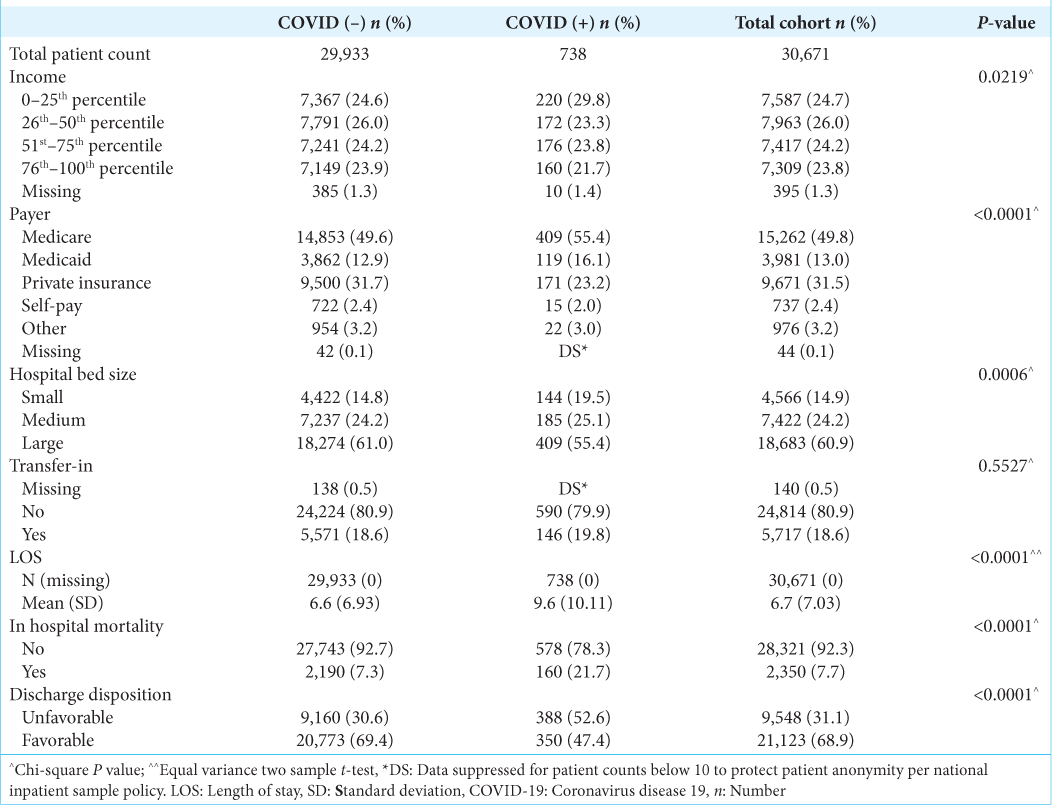
COVID-19 patients who underwent surgical resection for malignant brain tumors trended toward a longer time from admission to surgery (3.1 days for COVID-19 patients vs. 2.1 days for patients without COVID-19); however, this finding was not statistically significant (P = 0.17). More COVID-19 patients also had 3 or more days from admission to surgery compared to patients without COVID-19 infection (≥3, 42.9% vs. 39.0%), but this difference was also not statistically significant (P = 0.25).
Patient outcomes
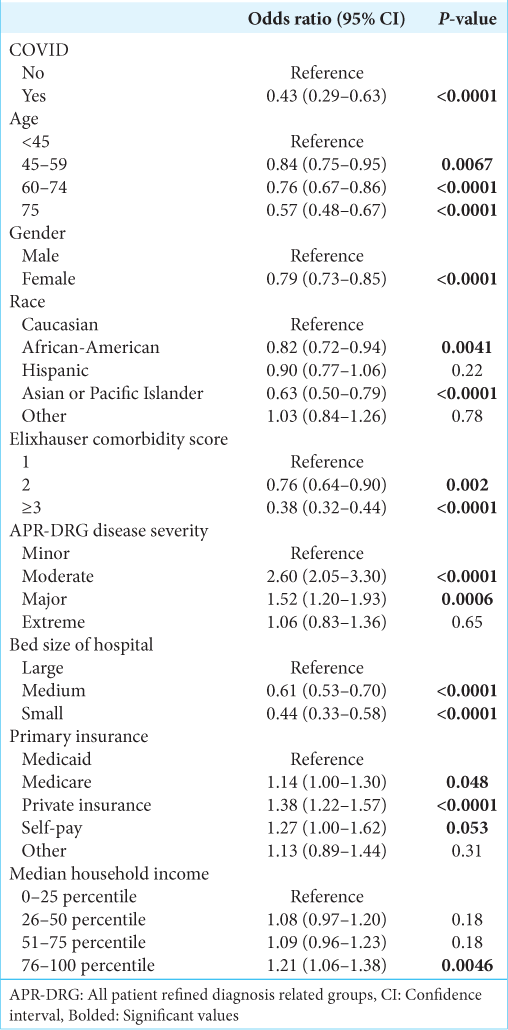
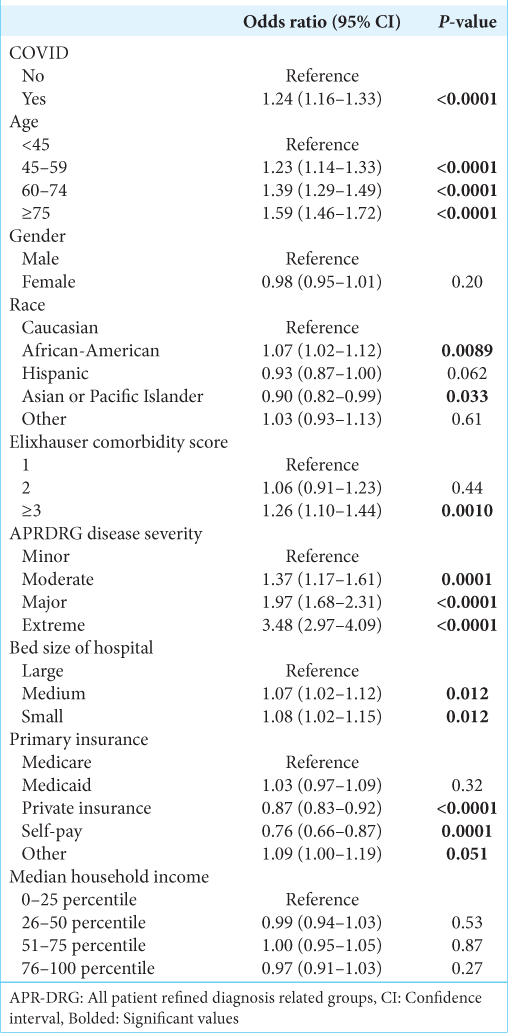
After controlling for other covariates, COVID-19 infection was significantly associated with a decreased likelihood of receiving surgery (Odds ratio [OR] 0.43, 95% confidence interval [CI] 0.29–0.63, P < 0.0001) [
Sub-cohort analysis of malignant primary tumors and malignant secondary (metastatic) tumors demonstrated similar results. In patients with malignant primary tumors, COVID-19 infection was associated with a decreased likelihood of receiving surgery (OR 0.37, 95% CI 0.22–0.62, P < 0.0002), increased likelihood of mortality (OR 1.91, 95% CI 1.15–3.16, P = 0.01), and increased likelihood of non-routine discharge (OR 1.19, 95% CI 1.01–1.40, P = 0.04). In patients with malignant secondary tumors, COVID-19 infection was associated with a decreased likelihood of receiving surgery (OR 0.37, 95% CI 0.20–0.68, P = 0.001), increased likelihood of mortality (OR 2.36, 95% CI 1.88– 2.96, P < 0.0001), and increased likelihood of non-routine discharge (OR 1.24, 95% CI 1.08–1.43, P = 0.002).
Additional characteristics associated with likelihood of receiving surgery are shown in
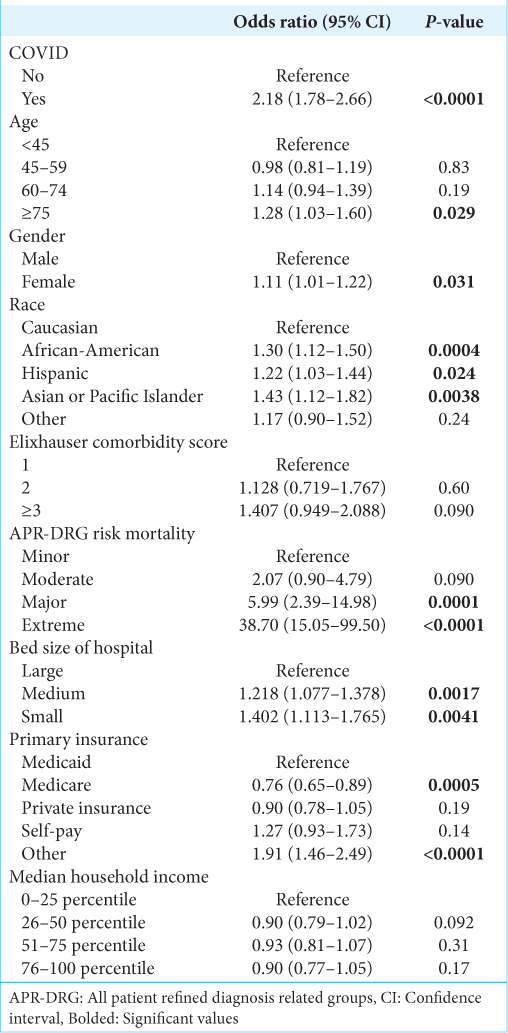
The factors associated with increased likelihood of mortality are shown in
Factors associated with likelihood of non-routine discharge are shown in
DISCUSSION
In our analysis of the NIS, malignant brain tumor patients with COVID-19 were associated with a decreased likelihood of receiving surgery, increased likelihood of in-hospital mortality, and increased likelihood of non-routine discharge. Notably, despite a decreased likelihood of surgery among COVID patients, those COVID patients receiving surgery did not demonstrate an association with surgical delay. To date, this study is the first to identify these findings using a large, national dataset and the findings of our study provide important insight into optimal management of these patients in resource-scarce circumstances like the COVID-19 pandemic.
Surgical resection is an essential component in the treatment of many malignant brain tumors. However, during the COVID-19 pandemic, many hospitals had to postpone or cancel non-emergent surgeries due to concerns about the risk of infection and need to preserve hospital resources. While often necessary, malignant tumor resections are typically not “emergent” and, as such, were often classified as “elective.” However, due to the aggressive nature of malignant brain tumors, delaying surgery up to 7 weeks – as prior guidelines have recommended – is not always feasible.[
In addition, our study found that patients with concurrent COVID-19 infection had 2.18 times greater odds of mortality than those without COVID-19. Patients with both cancer and COVID-19 infection pose a unique clinical dilemma – in the interest of not advancing their COVID-19 infection, it may be sensible to withhold chemotherapy and steroid treatment so as to not further immunosuppress these patients.[
Further analysis is necessary to elucidate the etiology of increased mortality among patients with brain tumors who have COVID-19. Intrinsic medical factors, such as cytokine storm, neuroinflammation, and endothelial cell destruction secondary to COVID-19 infection, could contribute to worse outcomes in brain tumor patients.[
Our analysis also found demographic differences between patients with malignant brain tumors with and without COVID-19. A higher percentage of malignant brain tumor patients with COVID-19 were African-American (14.9%) and Hispanic (14.5%) compared to patients without COVID-19 (12% were African-American and 7.2% were Hispanic). Racial disparities in COVID-19 infections and outcomes have been well documented in the United States; African-American and Hispanic populations have an estimated 1.5–3.5 higher risk and 1.3–7.7 higher risk, respectively, compared to white populations.[
Limitations
Our retrospective and cohort study, which utilized the NIS, has several inherent limitations. Although the NIS contains a large number of patients, only 1 year of 2020 data has been made public so far, limiting the number of documented COVID-19 patients who underwent tumor resection. The analysis was therefore underpowered, which may affect the statistical significance noted in assessing the association of COVID with time to surgery. The NIS also cannot identify patients with multiple hospitalizations, resulting in readmission being recorded as new patients. This limits the ability to track patients with long-term conditions and assess for long-term cancer survival. Furthermore, the NIS does not distinguish pre-existing conditions and hospital-acquired conditions. In addition, the NIS is specific to the United States, and since the impact of the COVID-19 pandemic varies greatly across different regions and countries, NIS data may not be generalizable. Finally, as with any administrative database, reliance on ICD-10 coding can result in errors and subjectivity in data input and coding. Despite these limitations, our study provides valuable insights into the relationship between malignant brain tumors and COVID-19. Further research is needed to validate our findings and to address the limitations of the NIS.
CONCLUSION
Overall, malignant brain tumor patients with COVID-19 infection were associated with worse overall outcomes, including decreased likelihood of receiving surgery, increased likelihood of mortality, and increased likelihood of non-routine discharge. To the best of our knowledge, this study is the first to analyze the relationship of these outcomes with COVID-19 infection with a large national dataset. The findings of our study emphasize the need to balance the risks and benefits of delaying surgery for malignant brain tumor patients with COVID-19. Further study on causal relationships between COVID-19 and patient outcomes is needed to optimize management for this patient population at risk for direct effects of COVID infection and indirect health-care system consequences of COVID-19. As such, though the COVID-19 pandemic is no longer a public health emergency, reinforcing our understanding of the pandemic’s impact on outcome provides important insight in effective triage for these patients in future situations where resources are limited.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Li Ding was supported by grants UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY TABLE
Acknowledgments
Li Ding was supported by grants UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: An international collaborative group. Oncologist. 2020. 25: e936-45
2. Anesi GL, Lynch Y, Evans L. A conceptual and adaptable approach to hospital preparedness for acute surge events due to emerging infectious diseases. Crit Care Explor. 2020. 2: e0110
3. ASA and APSF Joint Statement on Elective Surgery/Procedures and Anesthesia for Patients after COVID-19 Infection. Available from: https://www.apsf.org/wp-content/uploads/news-updates/2022/ASA-APSF-Joint-Statement-Elective-Surgery-2022-02-22.pdf [Last accessed on 2023 Mar 07].
4. Azar KM, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood). 2020. 39: 1253-62
5. Burke JF, Chan AK, Mummaneni V, Chou D, Lobo EP, Berger MS. Letter: The coronavirus disease 2019 global pandemic: A neurosurgical treatment algorithm. Neurosurgery. 2020. 87: E50-6
6. Chai C, Feng X, Lu M, Li S, Chen K, Wang H. One-year mortality and consequences of COVID-19 in cancer patients: A cohort study. IUBMB Life. 2021. 73: 1244-56
7. Collaborative CO. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br J Surg. 2020. 107: 1440-9
8. COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. Available from: https://www.facs.org/formedical-professionals/covid-19/clinical-guidance/triage [Last accessed on 2023 Mar 07].
9. de Joode K, Taal W, Snijders TJ, Hanse M, Koekkoek JA, Oomen-de Hoop E. Patients with primary brain tumors and COVID-19: A report from the Dutch Oncology COVID-19 Consortium. Neuro Oncol. 2022. 24: 326-8
10. Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020. 26: 402-14
11. Kendzerska T, Zhu DT, Gershon AS, Edwards JD, Peixoto C, Robillard R. The effects of the health system response to the COVID-19 pandemic on chronic disease management: A narrative review. Risk Manag Healthc Policy. 2021. 14: 575-84
12. Kessler RA, Zimering J, Gilligan J, Rothrock R, McNeill I, Shrivastava RK. Neurosurgical management of brain and spine tumors in the COVID-19 era: An institutional experience from the epicenter of the pandemic. J Neurooncol. 2020. 148: 211-9
13. Luo C, Song K, Wu S, Hameed NU, Kudulaiti N, Xu H. The prognosis of glioblastoma: A large, multifactorial study. Br J Neurosurg. 2021. 35: 555-61
14. Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: A systematic review. Ann Intern Med. 2021. 174: 362-73
15. Magesh S, John D, Li WT, Li Y, Mattingly-App A, Jain S. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: A systematic-review and meta-analysis. JAMA Netw Open. 2021. 4: e2134147
16. Marenco-Hillembrand L, Erben Y, Suarez-Meade P, Franco-Mesa C, Sherman W, Eidelman BH. Outcomes and surgical considerations for neurosurgical patients hospitalized with COVID-19-A multicenter case series. World Neurosurg. 2021. 154: e118-29
17. McCain JL, Wang X, Connell K, Morgan J. Assessing the impact of insurance type on COVID-19 mortality in black and white patients in the largest healthcare system in the state of georgia. J Natl Med Assoc. 2022. 114: 218-26
18. Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020. 6: 495-503
19. National (Nationwide) Inpatient Sample (NIS) Database Documentation. Available from: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp [Last accessed on 2023 Mar 05].
20. NC. Guidelines Version 2.2022. 2022. p. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Last accessed on 2023 Mar 07]
21. Newson G, Gilbert BP, editors. Medi-Cal Guidance Relating to Non-Urgent, Non-Essential, or Elective Procedures Relative to the 2019 Novel Coronavirus (COVID-19). United States: Department of Health Care Services, State of California Health and Human Services Agency; 2020. p.
22. Non-Emergent. Elective Medical Services, and Treatment Recommendations. Available from: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf [Last accessed on 2023 Jul 23].
23. Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022. 24: v1-95
24. Price SJ, Joannides A, Plaha P, Afshari FT, Albanese E, Barua NU. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: A national survey (COVID-CNSMDT Study). BMJ Open. 2020. 10: e040898
25. Qi X, Keith KA, Huang JH. COVID-19 and stroke: A review. Brain Hemorrhages. 2021. 2: 76-83
26. Samer Zammar SS. COVID-19 and Neurosurgery. Available from: https://www.aans.org/en/patients/neurosurgical-conditions-and-treatments/covid-19-andNeurosurgery [Last accessed on 2023 Mar 07].
27. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
28. Sarpong K, Dowlati E, Withington C, Chesney K, Mualem W, Hay K. Perioperative coronavirus disease 2019 (COVID-19) incidence and outcomes in neurosurgical patients at two tertiary care centers in Washington, DC, during a Pandemic: A 6-month follow-up. World Neurosurg. 2021. 146: e1191-201
29. Sarwan G, Mubarak T, Puello P, Brisman M, Grewal J. Negative impact of COVID-19 upon primary brain tumor care. Cureus. 2021. 13: e17800
30. SEER Cancer Statistics Review 1975-2018 [database on the Internet]. Available from: https://seer.cancer.gov/archive/csr/1975_2018 [Last accessed on 2023 Mar 06].
31. SEER Surveillance, Epidemiology, and End Results Program (SEER Database). Available from: https://seer.cancer.gov/statfacts/html/brain.html [Last accessed on 2023 Mar 05].
32. Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020. 31: 1065-74
33. Tai DB, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021. 72: 703-6
34. Weller M, Preusser M. How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open. 2020. 4: e000789
35. Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP. Glioblastoma in adults: A Society for NeuroOncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020. 22: 1073-113
36. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Last accessed on 2023 Mar 07].
37. Wiesner SM, Freese A, Ohlfest JR. Emerging concepts in glioma biology: Implications for clinical protocols and rational treatment strategies. Neurosurg Focus. 2005. 19: E3
38. Zarifkar P, Kamath A, Robinson C, Morgulchik N, Shah SF, Cheng TK. Clinical characteristics and outcomes in patients with COVID-19 and cancer: A systematic review and meta-analysis. Clin Oncol (R Coll Radiol). 2021. 33: e180-91