- Department of Neurosurgery, Rush University Medical Center, Chicago, Illinois, USA
- Department of Otolaryngology – Head and Neck Surgery, Rush University Medical Center, Chicago, Illinois, USA
- Department of Neurology, Rush University Medical Center, Chicago, Illinois, USA
Correspondence Address:
Sepehr Sani
Department of Neurosurgery, Rush University Medical Center, Chicago, Illinois, USA
DOI:10.4103/sni.sni_412_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daniel Eddelman, Joshua Wewel, R. Mark Wiet, Leo V. Metman, Sepehr Sani. Deep brain stimulation with a pre-existing cochlear implant: Surgical technique and outcome. 05-Apr-2017;8:47
How to cite this URL: Daniel Eddelman, Joshua Wewel, R. Mark Wiet, Leo V. Metman, Sepehr Sani. Deep brain stimulation with a pre-existing cochlear implant: Surgical technique and outcome. 05-Apr-2017;8:47. Available from: http://surgicalneurologyint.com/surgicalint-articles/deep-brain-stimulation-with-a-pre%e2%80%91existing-cochlear-implant-surgical-technique-and-outcome/
Abstract
Background:Patients with previously implanted cranial devices pose a special challenge in deep brain stimulation (DBS) surgery. We report the implantation of bilateral DBS leads in a patient with a cochlear implant. Technical nuances and long-term interdevice functionality are presented.
Case Description:A 70-year-old patient with advancing Parkinson's disease and a previously placed cochlear implant for sensorineural hearing loss was referred for placement of bilateral DBS in the subthalamic nucleus (STN). Prior to DBS, the patient underwent surgical removal of the subgaleal cochlear magnet, followed by stereotactic MRI, frame placement, stereotactic computed tomography (CT), and merging of imaging studies. This technique allowed for successful computational merging, MRI-guided targeting, and lead implantation with acceptable accuracy. Formal testing and programming of both the devices were successful without electrical interference.
Conclusion:Successful DBS implantation with high resolution MRI-guided targeting is technically feasible in patients with previously implanted cochlear implants by following proper precautions.
Keywords: Cochlear implant, deep brain stimulation, magnetic resonance imaging, Parkinson disease
INTRODUCTION
Deep brain stimulation (DBS) has become an increasingly acceptable treatment modality for treatment of Parkinson's disease (PD),[
Given the aging population, a neurosurgeon is likely to see DBS candidates that have other previously implanted electronic devices. Safe implantation of DBS in patients with cardiac pacemakers has been described.[
The presence of a pre-existing cochlear implant presents a number of challenges when considering DBS placement. The implant magnet is not MRI compatible, necessitating its removal surgically or alternatively using non-MRI based imaging for DBS targeting. If the magnet is removed, there is loss of hearing, and henceforth communication with the patient is diminished during DBS surgery.
We describe successful bilateral STN DBS implantation using MRI-based targeting and intraoperative CT (iCT) utilization in a patient with a pre-existing cochlear implant. Targeting accuracy and nuances leading to a safe and reliable function of both implanted devices are discussed.
CASE REPORT
Clinical background
The patient is a 70-year-old right-handed male with a 14-year history of PD. He also had a right posterior temporal cochlear implant (Advanced Bionics 90K, Valencia, CA) placed 2 years ago due to sensorineural hearing loss. After evaluation by the surgical movement disorders team, he was determined to be an appropriate candidate for bilateral STN DBS.
Surgical technique
Cochlear implant magnet removal
The surgical care was coordinated with the neurotology team. One day prior to DBS implantation, the patient was taken to the operating room by the neurotology team and placed under general anesthesia. The receiver stimulator portion of the implant was marked off by using the patient's transmitting coil as a stencil, and a half circle was drawn along the top of the receiver stimulator portion of the implant. After incision, a subgaleal pocket was developed over the cochlear implant. The soft tissue overlying the receiver implant was opened. While being careful to not damage cochlear implant, the capsule overlying the implant itself was opened in the same manner. The cochlear implant magnet was identified, removed from its pocket, and the wound was closed.
Deep brain stimulation surgery
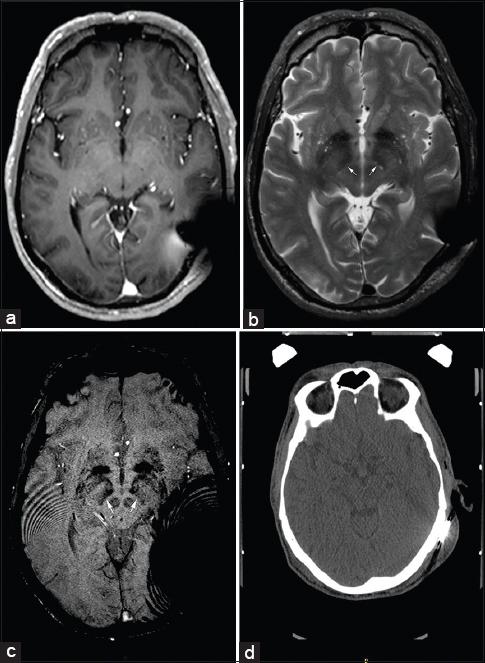
After magnet removal, preoperative stereotactic MRI of brain was performed (T1 post-contrast volumetric, T2 axial, and SWI axial sequences) [
Figure 1
MRI and CT images of patient with cochlear implant after magnet removal demonstrating various degrees of signal artifact. (a) T1W axial section demonstrates artifact extending to the subcortical white matter of posterior temporal lobe. (b and c) T2W and SWI sequences, respectively, showing undistorted anatomy of subthalamic nucleus and midbrain structures. Arrows show medial STN borders. (d) Axial noncontrast CT with normal visualization of fiducial markers on the localizer
Targeting was done using the Framelink 5.1 software (Medtronic, Minneapolis, MN). Anatomic locations of anterior commissure (AC), posterior commissure (PC), and midline markers were defined to create images orthogonal to the AC–PC plane and mid-sagittal plane. Of note, both STN and globus pallidus pars interna (GPi) were successfully visualized on T2 and SWI sequences [
The patient was positioned supine on the operating table in the “recline” position. Details of positioning using iCT for DBS have been previously described.[
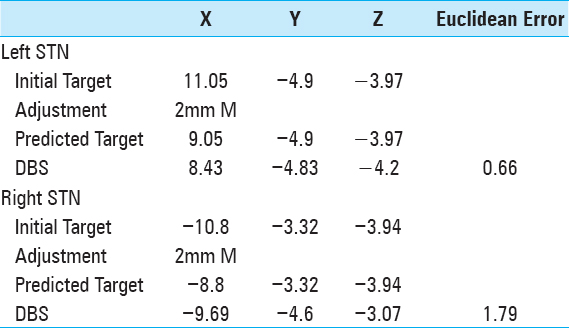
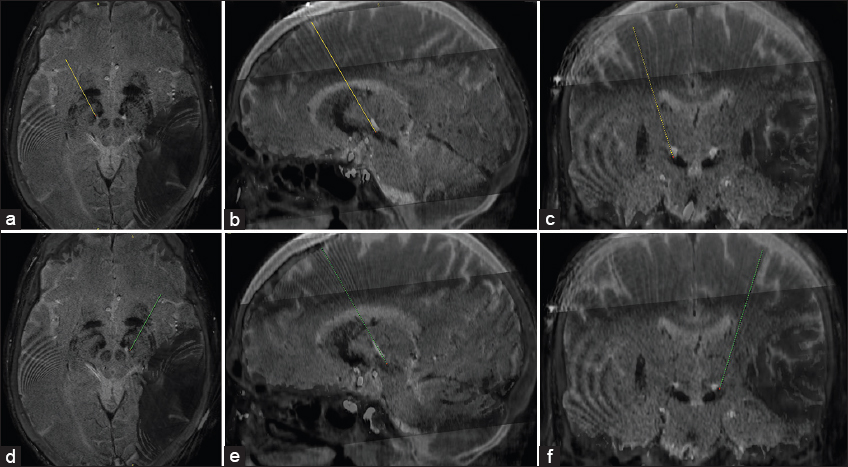
After draping, the Leksell arc and headstage were assembled. Burr holes were made. A 1.27 mm diameter and 25 mm length sterile guide tube (Alpha Omega, Nazareth, Israel) was inserted to a depth of 15 mm above target. Microelectrode recording ensued at this time. The leads (model 3389, Medtronic, Inc., Minneapolis, Minnesota) were subsequently assembled and advanced to target. iCT images were obtained and computationally merged with the preoperative CT [
Cochlear implant magnet replacement
Immediately following DBS implantation, the patient was placed under conscious sedation. The postauricular area was prepped and draped. The incision that was made for cochlear implant removal 24 hours earlier was opened, the receiver stimulator portion of the cochlear implant was identified, and a new cochlear implant magnet was placed. The wound was closed in layers. The patient recovered uneventfully and was discharged home the following day.
One week later, he presented for placement of the pulse generator in the left chest and connection to the DBS leads.
Postoperative course
There were no surgical or postoperative complications. The incision for cochlear implant magnet removal was allowed to heal for 2 weeks, at which point the sutures were removed. The patient was asked not to use the cochlear implant for an additional 2 weeks to avoid infection of this wound and potential contamination of the cochlear implant. The patient's hearing and speech perception remained stable at 1 year after DBS implantation [
One month after the surgery the patient returned to the Movement Disorder clinic for initial DBS programming. Interrogation of the device revealed normal function without interference from the cochlear implant when tested at both monopolar and bipolar settings. Impedances were normal. Optimal clinical improvement was noted with bipolar settings (1- 2+ left; 9- 10+ right) at a voltage of 3.0 V, pulse width of 60 μs, and frequency of 130 Hz bilaterally. At these settings, the patient had significant tremor suppression with no adverse effects. At the latest follow-up 8 months after DBS implantation, the left side voltage was increased to 3.2 with maintained efficacy.
DISCUSSION
The presented case suggests that direct targeting of the basal ganglia and thalamic nuclei is feasible and safe using MRI in a patient with a cochlear implant. Safe implantation of DBS in patients with cardiac pacemakers[
In the presented report, we were able to circumvent the abovementioned issues by close temporal interval removal of the cochlear magnet, obtaining the MRI study before frame placement, with next day frame placement, and CT image merging for targeting. The accuracy of MRI–CT merging has been previously described and validated.[
While the presented technique offers better anatomical resolution for planning, it has the drawback of requiring the orchestration of multiple procedures in close proximity, along with the associated potential increased risks such as infection.
Functionality of the DBS device and clinical efficacy of either device do not appear to be affected by a pre-existing cochlear implant given the clinical results and normal impedances at the latest follow-up. This is consistent with previous case reports.[
The cochlear implant functionality appears to remain intact with no change in hearing function after DBS surgery, as shown here. This is likely due to the low level of stimulation and spatial separation of the devices. Another important issue of consideration is patient communication after magnet removal, especially during DBS surgery. Buell et al.[
CONCLUSION
Implantation of a nonrechargeable open loop DBS system in patients with pre-existing cochlear implants appears to be safe.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Altinay M, Estemalik E, Malone DA. A Comprehensive Review of the Use of Deep Brain Stimulation (DBS) in Treatment of Psychiatric and Headache Disorders. Headache. 2015. 55: 345-50
2. Aviles-Olmos I, Kefalopoulou Z, Tripoliti E, Candelario J, Akram H, Martinez-Torres I. Long-term outcome of subthalamic nucleus deep brain stimulation for Parkinson's disease using an MRI-guided and MRI-verified approach. J Neurol Neurosurg Psychiatr. 2014. 85: 1419-25
3. Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatr. 2014. 85: 567-72
4. Buell TJ, Ksendzovsky A, Shah BB, Kesser BW, Elias JW. Deep brain stimulation in the setting of cochlear implants: Case report and literature review. Stereot Funct Neurosurg. 2015. 93: 245-9
5. Capelle HH, Simpson RK, Kronenbuerger M, Michaelsen J, Tronnier V, Krauss JK. Long-term deep brain stimulation in elderly patients with cardiac pacemakers. J Neurosurg. 2005. 102: 53-9
6. Cif L, Gonzalez V, Garcia-Ptacek S, James S, Boetto J, Seychelles A. Progressive dystonia in Mohr-Tranebjaerg syndrome with cochlear implant and deep brain stimulation. Mov Disord. 2013. 28: 737-8
7. De Los Reyes K, Chandrasekhar SS, Tagliati M, Alterman R. Successful implantation of a deep brain stimulator for essential tremor in a patient with a preexisting cochlear implant: Surgical technique: Technical case report. Neurosurgery. 2010. 66: 372-
8. Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson's disease. Handb Clin Neurol. 2013. 116: 107-28
9. Kalia SK, Sankar T, Lozano AM. Deep brain stimulation for Parkinson's disease and other movement disorders. Curr Opin Neurol. 2013. 26: 374-80
10. Lilleeng B, Brønnick K, Toft M, Dietrichs E, Larsen JP. Progression and survival in Parkinson's disease with subthalamic nucleus stimulation. Acta Neurol Scand. 2014. 130: 292-8
11. Mirzadeh Z, Chapple K, Lambert M, Dhall R, Ponce FA. Validation of CT-MRI fusion for intraoperative assessment of stereotactic accuracy in DBS surgery. Mov Disord. 2014. 29: 1788-95
12. Nazzaro JM, Lyons KE, Pahwa R. Deep brain stimulation for essential tremor. Handb Clin Neurol. 2013. 116: 155-66
13. Ngoga D, Mitchell R, Kausar J, Hodson J, Harries A, Pall H. Deep brain stimulation improves survival in severe Parkinson's disease. J Neurol Neurosurg Psychiatr. 2014. 85: 17-22
14. O’Gorman RL, Jarosz JM, Samuel M, Clough C, Selway RP, Ashkan K. CT/MR image fusion in the postoperative assessment of electrodes implanted for deep brain stimulation. Stereotact Funct Neurosurg. 2009. 87: 205-10
15. Odekerken VJJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): A randomised controlled trial. Lancet Neurol. 2013. 12: 37-44
16. Pedrosa DJ, Auth M, Eggers C, Timmermann L. Effects of low-frequency thalamic deep brain stimulation in essential tremor patients. Exp Neurol. 2013. 248: 205-12
17. Peng-Chen Z, Morishita T, Vaillancourt D, Favilla C, Foote KD, Okun MS. Unilateral thalamic deep brain stimulation in essential tremor demonstrates long-term ipsilateral effects. Parkinsonism Relat Disord. 2013. 19: 1113-7
18. Pezeshkian P, DeSalles AAF, Gorgulho A, Behnke E, McArthur D, Bari A. Accuracy of frame-based stereotactic magnetic resonance imaging vs frame-based stereotactic head computed tomography fused with recent magnetic resonance imaging for post implantation deep brain stimulator lead localization. Neurosurgery. 2011. 69: 1299-306
19. Sarubbo S, Latini F, Quatrale R, Sensi M, Granieri E, Cavallo MA. Five-year follow-up of 10 patients treated with globus pallidus internus deep brain stimulation for segmental or multisegmental dystonia. Stereotact Funct Neurosurg. 2012. 90: 84-91
20. Senatus PB, McClelland S, Ferris AD, Ford B, Winfield LM, Pullman SL. Implantation of bilateral deep brain stimulators in patients with Parkinson disease and preexisting cardiac pacemakers. Report of two cases. J Neurosurg. 2004. 101: 1073-7
21. Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: Technique and accuracy assessment. Neurosurgery. 2011. 68: 114-24
22. Shin M, Lefaucheur JP, Penholate MF, Brugières P, Gurruchaga JM, Nguyen JP. Subthalamic nucleus stimulation in Parkinson's disease: Postoperative CT-MRI fusion images confirm accuracy of electrode placement using intraoperative multi-unit recording. Neurophysiol Clin. 2007. 37: 457-66
23. Smith AP, Bakay RAE. Frameless deep brain stimulation using intraoperative O-arm technology. Clinical article. J Neurosurg. 2011. 115: 301-9
24. St Martin MB, Hirsch BE. Cochlear implantation in a patient with bilateral deep brain stimulators. Laryngoscope. 2007. 117: 183-5
25. Vidailhet M, Jutras MF, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatr. 2013. 84: 1029-42
26. Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology. 2012. 79: 55-65
27. Williams NR, Okun MS. Deep brain stimulation (DBS) at the interface of neurology and psychiatry. J Clin Invest. 2013. 123: 4546-56