- Department of Neurosurgery, Osaka City University Graduate School of Medicine, 1-4-3 Asahi-machi, Abeno-ku, Osaka 545 - 8585, Japan
- Department of Neurosurgery, Yao Tokushukai General Hospital, 1-11 Wakakusa-cho, Yao city, Osaka, 581 - 0011, Japan
Correspondence Address:
Junya Abe
Department of Neurosurgery, Osaka City University Graduate School of Medicine, 1-4-3 Asahi-machi, Abeno-ku, Osaka 545 - 8585, Japan
DOI:10.4103/2152-7806.157792
Copyright: © 2015 Abe J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Abe J, Ichinose T, Terakawa Y, Tsuyuguchi N, Tsuruno T, Ohata K. Efficacy of arachnoid plasty with collagen sheets and fibrin glue: An in vitro experiment and a case review. Surg Neurol Int 28-May-2015;6:90
How to cite this URL: Abe J, Ichinose T, Terakawa Y, Tsuyuguchi N, Tsuruno T, Ohata K. Efficacy of arachnoid plasty with collagen sheets and fibrin glue: An in vitro experiment and a case review. Surg Neurol Int 28-May-2015;6:90. Available from: http://surgicalneurologyint.com/surgicalint_articles/efficacy-arachnoid-plasty-collagen-sheets-fibrin-glue/
Abstract
Background:Postoperative subdural fluid collection sometimes occurs after clipping of cerebral aneurysms. Arachnoid plasty is used to prevent such postoperative complications; however, the optimal materials for arachnoid plasty remain unclear. In this study, we aimed to clarify the optimal materials for arachnoid plasty and report our experience of arachnoid plasty after clipping of unruptured aneurysms.
Methods:In an in vitro experiment, adhesive strengths of three materials permitted for use in the intradural space, such as collagen sheets, gelatin sponge, and oxidized cellulose sheets, were measured by assessing their water pressure resistance. Then, 80 consecutive cases surgically treated unruptured cerebral aneurysms were retrospectively reviewed to examine the occurrence rate of postoperative subdural fluid collection.
Results:The collagen sheet exhibited the greatest adhesive strength, so we used collagen sheets for the arachnoid plasty procedures. In all of these cases, arachnoid plasty was performed with fibrin glue-soaked collagen sheets. No postoperative subdural fluid collection, inflammation, or allergic reactions occurred in any case.
Conclusions:The present study suggests that collagen sheet might be one of the optimal materials for arachnoid plasty. This technique is simple and may be effective to prevent subdural fluid collection after clipping.
Keywords: Arachnoid plasty, collagen sheets, water pressure resistance
INTRODUCTION
Postoperative subdural fluid collection (SFC) is a complication of craniotomy and incidence of 6–51% is reported.[
MATERIALS AND METHODS
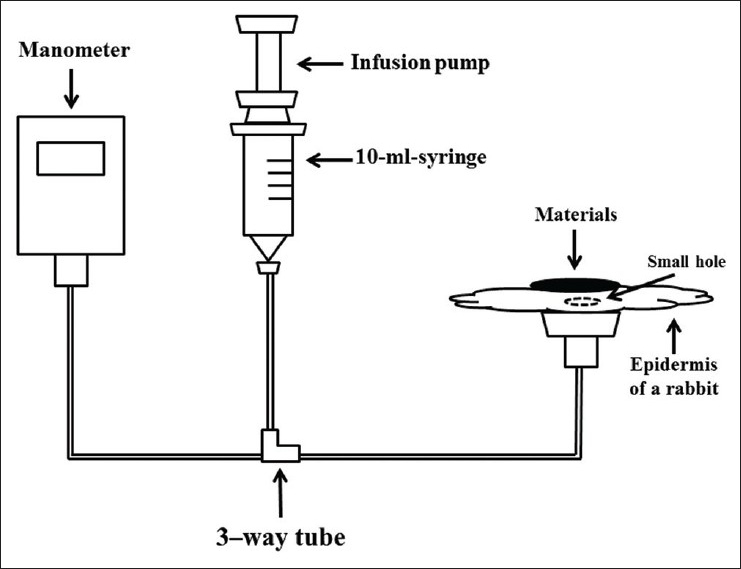
In vitro experiment of water pressure resistant
The following three materials, which are often used in neurosurgery to achieve hemostasis or strengthen suture points and are permitted for use in the intradural space, had their water pressure resistance assessed in the present study: Collagen sheets (INTEGRAN press sheet-type, Nippon Zoki Pharmaceutical Co., Ltd., Tokyo), oxidized cellulose sheets (SURGICEL NU-KNIT, Johnson and Johnson K.K., Tokyo), and gelatin sponge (Gelfoam, Pfizer Inc., Tokyo).
The experimental protocol is as follows and the scheme of experiment is shown in [
Patient population of arachnoid plasty
Between September 2009 and March 2013, there were 80 consecutive cases in which clipping surgery of unruptured cerebral aneurysms of the anterior circulation was performed at the Department of Neurosurgery, Yao Tokushukai General Hospital. There were 25 male and 55 female, mean age was 65.8 ± 9.4 years (mean ± SD).
Regarding the locations of the aneurysms, 32 aneurysms originated from the internal carotid artery (ICA), 30 from middle cerebral artery (MCA), and 18 from anterior communicating artery (A-Com) or anterior cerebral artery (ACA). Most of the clipping procedures were performed via a transsylvian approach. An interhemispheric approach was selected in four cases in which the aneurysms were located in the A-Com or distal ACAs. In all cases, the arachnoid plasty was performed with fibrin glue-soaked collagen sheets.
Surgical technique
In the transsylvian approach, the Sylvian fissure was dissected, and the arachnoid trabeculae were cut between the frontal and temporal lobes from deep within the Sylvian fissure to its surface. To minimize brain retraction, the interhemispheric fissure and contralateral cistern were opened in some cases. After the clipping had been performed, arachnoid plasty was conducted. Collagen sheets that had been soaked with fibrin glue were used to cover the exposed arachnoid space extended to anterior skull base, and then solution B was dripped onto them. Collagen sheets were shaped rectangular and covered both the Sylvian fissure and anterior skull base. Artificial cerebrospinal fluid (CSF) was then injected into the Sylvian fissure through a drainage tube. As a result, the brain, which shrank during clipping surgery, gradually expanded. Subsequently, we removed the drainage tube and covered the hole with the collagen sheets. Lastly, we closed the dura in a water tight fashion.
In the interhemispheric approach, the arachnoid plasty is performed after clipping in the same fashion. The exposed arachnoid space and anterior skull base were covered with the collagen sheets soaked fibrin glue, and the artificial CSF was injected into the interhemispheric fissure.
RESULTS
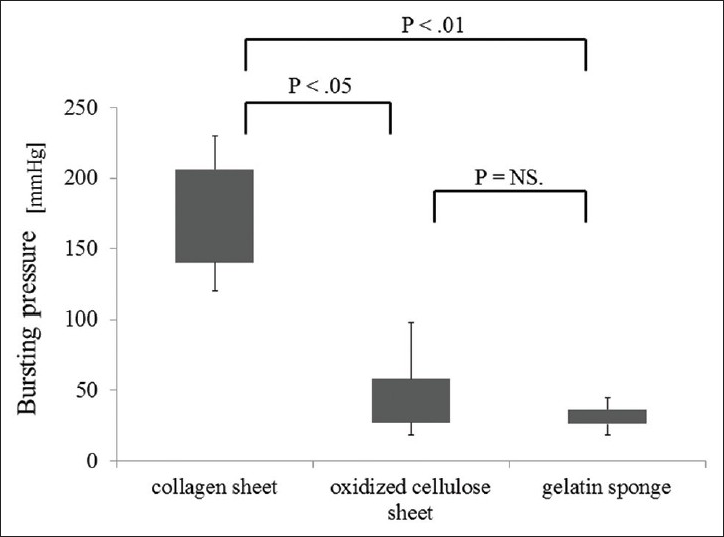
In vitro experiment of water pressure resistant
The results of the water pressure resistance experiment are shown in [
Results of arachnoid plasty
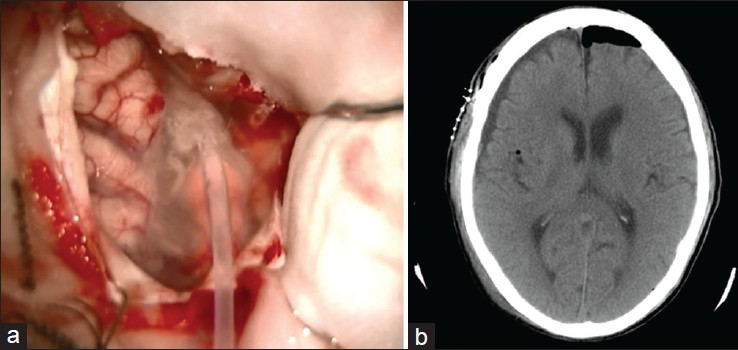
The median follow-up period was 21 months (range, 1–42 months). SFC was diagnosed based on brain computed tomography (CT) scans obtained about 1 month after surgery. Subdural spaces of less than 5 mm in diameter were not considered to be indicative of SFC. In this study, SFC did not occur in any case, nor did infections or allergic reactions. The illustrative case is shown in
DISCUSSION
Arachnoid plasty has been demonstrated to be effective for preventing postoperative SFC.[
The efficacy of arachnoid plasty with collagen sheet and fibrin glue after the clipping of unruptured aneurysms was examined. As a result, we found that the procedure achieved good outcomes, and there were no incidence of SFC or complications such as surgical infection. The leakage of CSF and brain shrinkage is considered to be the main causes of SFC after the opening of the arachnoid space.[
Collagen sheets demonstrated strong adhesiveness in our in vitro experiment, and, in fact, were found to be useful for preventing postoperative SFC after clipping surgery of unruptured aneurysms. Additionally, precise manipulation is required due to the limited surgical field in neurosurgical procedures. Therefore, the materials for arachnoid plasty must not only display resistance to water pressure but also be easy to manipulate. This feature allowed us to cover even deep parts of the brain such as the anterior skull base. Accordingly, we consider that a combination of collagen sheets and fibrin glue might be one of the suitable techniques for arachnoid plasty and feasible for preventing postoperative SFC.
Limitations
In this study, arachnoid plasty performed with collagen sheets and fibrin glue achieved favorable results. However, we assessed the water pressure resistance of the examined materials just 3 min after the fibrin glue had been applied. It is possible that the water resistance of the materials changes gradually over time. Another limitation is that the fibrin glue mixing ratio was not examined in this experiment. A previous in vitro study reported that a fibrin glue mixing ratio of A/B 5:1 resulted in significantly greater pressure resistance than a ratio of A/B 1:1.[
CONCLUSIONS
Collagen sheets soaked with fibrin glue, which display strong adhesiveness and water pressure resistance, are useful covering materials for arachnoid plasty. Arachnoid plasty with collagen sheets and fibrin glue is a safe technique and appears to be effective for preventing SFC after clipping of unruptured aneurysms.
ACKNOWLEDGMENTS
The authors thank Mr. Kenichiro Hirahashi, Kaketsuken, for his assistance in preparing our study. There was no financial support from Kaketsuken.
References
1. Al-Yamany M, Del Maestro RF. Prevention of subdural fluid collections following transcortical intraventricular and/or paraventricular procedures by using fibrin adhesive. J Neurosurg. 2000. 92: 406-12
2. Blauwblomme T, Harkness W. Corticotomy closure avoids subdural collections after hemispherotomy. Neurosurgery. 2010. 67: 485-8
3. Boyd MC, Steinbok P. Choroid plexus tumors: Problems in diagnosis and management. J Neurosurg. 1987. 66: 800-5
4. Hirsch JF, Sainte-Rose C. A new surgical approach to subcortical lesions: Balloon inflation and cortical gluing.Technical note. J Neurosurg. 1991. 74: 1014-7
5. Jung TY, Jung S, Jin SG, Jin YH, Kim IY, Kang SS. Prevention of postoperative subdural fluid collections following transcortical transventricular approach. Surg Neurol. 2007. 68: 172-6
6. Koizumi H, Fukamachi A, Nukui H. Postoperative subdural fluid collections in neurosurgery. Surg Neurol. 1987. 27: 147-53
7. Mervart M. Extracerebral fluid collection in infancy. J Neurosurg. 1995. 82: 148-9
8. Milhorat TH. Closure of cerebral incisions following intraventricular operations. Technical note. J Neurosurg. 1971. 35: 108-11
9. Mino Y, Hirashima Y, Hamada H, Masuoka T, Yamatani K, Takeda S. Effect of arachnoid plasty using fibrin glue membrane after clipping of ruptured aneurysm on the occurrence of complications and outcome in the elderly patients. Acta Neurochir (Wien). 2006. 148: 627-31
10. Moroi J, Hadeishi H, Suzuki A, Yasui N. Morbidity and mortality from surgical treatment of unruptured cerebral aneurysms at Research Institute for Brain and Blood Vessels-Akita. Neurosurgery. 2005. 56: 224-31
11. Murata K. Chronic subdural hematoma may be preceded by persistent traumatic subdural effusion. Neurol Med Chir (Tokyo). 1993. 33: 691-6
12. Nishimura S, Fujita T, Sakata H, Hori E, Mino M, Nishijima M. Chronic subdural hematoma and remote cerebellar hemorrhage developing after unruptured aneurysmal surgery. Surg Cereb Stroke. 2009. 37: 350-6
13. Nozaki K, Kikuta K, Takagi Y, Nishimura M. Surgical outcomes and complications of open surgery for unruptured cerebral aneurysms in anterior circulation. Surg Cereb Stroke. 2009. 37: 40-5
14. Orz YI, Hongo K, Tanaka Y, Nagashima H, Osawa M, Kyoshima K. Risks of surgery for patients with unruptured intracranial aneurysms. Surg Neurol. 2000. 53: 21-9
15. Raaymakers TW, Rinkel GJ, Limburg M, Algra A. Mortality and morbidity of surgery for unruptured intracranial aneurysms: A meta-analysis. Stroke. 1998. 29: 1531-8
16. Saitoh Y, Oshino S. Enhancement of withstanding pressure of fibrin sealant by modified mixing ratio of fibrin sealant components for skull base reconstruction: Technical note. Neurol Med Chir (Tokyo). 2013. 53: 65-8
17. Sawamura A, Kamiyama H, Kobayashi N, Makino K, Takizawa K, Yasuda H. A new technique for the prevention of brain collapse using fibrin glue and “Floating bypass” technique. Surg Cereb Stroke. 1999. 27: 479-82
18. Sawamura Y, Asaoka K, Terasaka S, Tada M, Uchida T. Evaluation of application techniques of fibrin sealant to prevent cerebrospinal fluid leakage: A new device for the application of aerosolized fibrin glue. Neurosurgery. 1999. 44: 332-7
19. Shaskey DJ, Mijer JF, Williams HJ, Sawitzke AD. Subdural fluid collections: An unusual manifestation of CNS disease in a connective tissue disorder. Clin Rheumatol. 1995. 14: 108-11
20. Tanaka Y, Mizuno M, Kobayashi S, Sugita K. Subdural fluid collection following craniotomy. Surg Neurol. 1987. 27: 353-6
21. Tanaka Y, Sugita K, Kobayashi S, Takemae T, Hegde AS. Subdural fluid collections following transcortical approach to intra- or paraventricular tumours. Acta Neurochir (Wien). 1989. 99: 20-5
22. Tashiro H, Morikawa A, Yamanaka M, Kuraishi K. Arachnoid closure making use of the fibrin membrane. Jpn J Neurosurg. 2000. 9: 775-7
23. Tsuruno T, Matsuoka Y, Hakuba A. An arachnoid plasty technique using a collagen seat and fibrin glue. Jpn J Neurosurg. 1995. 4: 193-5
24. Watanabe S, Shimada H, Ishii S. Production of clinical form of chronic subdural hematoma in experimental animals. J Neurosurg. 1972. 37: 552-61
25. Yamada H, Watanabe T, Murata S, Shibui S, Nihei H, Kohno T. Developmental process of chronic subdural collections of fluid based on CT scan findings. Surg Neurol. 1980. 13: 441-8
26. Yoshimoto T, Houkin K, Ishikawa T, Abe H. Arachnoid membrane closure. Prevention of postoperative cerebrospinal fluid leakage. Surg Neurol. 1999. 52: 68-72
27. Yoshimoto Y, Wakai S, Hamano M. External hydrocephalus after aneurysm surgery: Paradoxical response to ventricular shunting. J Neurosurg. 1998. 88: 485-9