- Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California,
- Department of Neurosurgery, Pacific Northwest University of Health Sciences, Yakima, Washington,
- Cedars-Sinai Medical Center, Spine Center, Los Angeles, California, United States.
Correspondence Address:
Terrence T. Kim, MD, Cedars-Sinai Medical Center, Spine Center, Los Angeles, California, United States.
DOI:10.25259/SNI_929_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Syed-Abdullah Uddin1, Katerina Roma2, Lindsey Ross1, George Hanna1, Doniel Drazin2, Terrence T. Kim3. Extensive spontaneous cervical epidural hematoma due to oral anticoagulant (dabigatran) successfully treated with reversal agent idarucizumab alone. 23-Jun-2022;13:259
How to cite this URL: Syed-Abdullah Uddin1, Katerina Roma2, Lindsey Ross1, George Hanna1, Doniel Drazin2, Terrence T. Kim3. Extensive spontaneous cervical epidural hematoma due to oral anticoagulant (dabigatran) successfully treated with reversal agent idarucizumab alone. 23-Jun-2022;13:259. Available from: https://surgicalneurologyint.com/surgicalint-articles/11666/
Abstract
Background: Dabigatran is an anticoagulant (novel oral anticoagulant) that is a direct thrombin inhibitor and only recently has a reversal agent, idarucizumab, been made available (2015).
Case Description: An 86-year-old male taking dabigatran for atrial fibrillation, acutely presented with the spontaneous onset of neck pain and quadriparesis. When the MRI demonstrated a C2-T2 spinal epidural hematoma, the patient was given the reversal agent idarucizumab. Due to his attendant major comorbidities, he was managed nonoperatively. Over the next 7 days, the patient’s neurological deficits resolved, and within 2 weeks, he had regained normal neurological function.
Conclusion: In this case, a C2-T2 epidural cervical hematoma attributed to dabigatran that was responsible for an acute, spontaneous quadriparesis was successfully treated with the reversal agent idarucizumab without surgical intervention being warranted.
Keywords: Anticoagulant reversal, Dabigatran, Idarucizumab, Pradaxa, Spinal epidural hematoma
INTRODUCTION
One of the first novel oral anticoagulants (NOACs) marketed was dabigatran. It is an oral direct thrombin inhibitor that has the longest half-life (12–17 h) of any of the direct anticoagulants (DOACs).[
CASE PRESENTATION
An 86-year-old male with atrial fibrillation presented with the acute onset of a spontaneous quadriparesis (i.e., upper extremities 2–3/5, lower extremities 5/5, diffuse hyperesthesia throughout the upper extremities, marked ataxia, and poor tandem gait). His medical history included idiopathic Parkinson’s disease of over 10 years duration. The patient’s medications included the anticoagulant dabigatran etexilate plus additional antiplatelet agents (i.e., clopidogrel and aspirin). His other medications included amiodarone, carbidopa-levodopa, and rasagiline.
Laboratory studies
Laboratory values revealed a serum WBC 4.8 × 103/uL, Hgb 13.0 g/dL, platelets 111 × 103/uL, an elevated PT (PT 14.3 s), and PTT (PTT 65 s). The INR was, however, just 1.0. Of interest, the troponin level was normal (0.01 ng/mL).
MR imaging
MRI sagittal imaging (i.e., could not tolerate complete sequences) demonstrated an expansile ventral epidural hematoma extending from C2 to T2 that resulted in significant cord compression. The signal within the clot was hypointense on T1 and hyperintense on the T2 sequences [
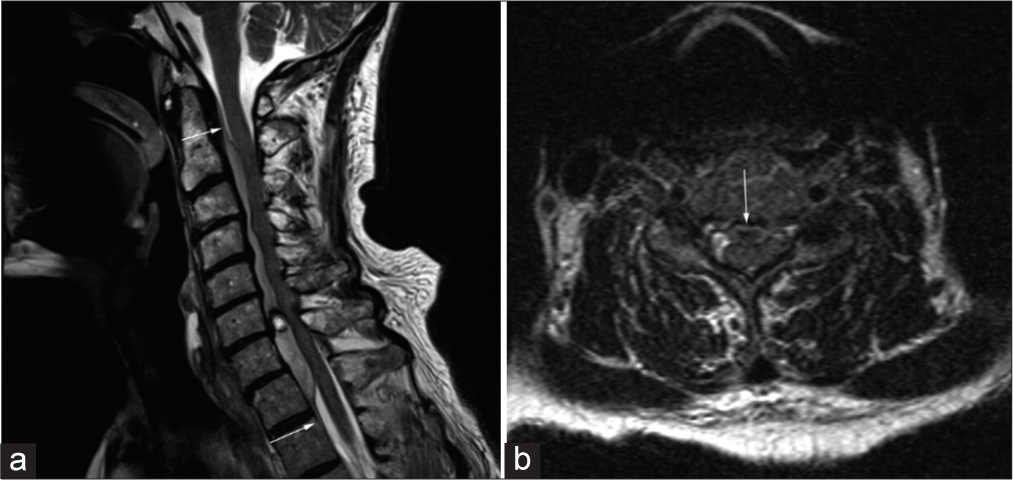
Figure 1:
Cervical spine MRI (a) T2-weighted sagittal view demonstrating extensive ventral epidural hematoma extending from the mid-body of C2 to the superior edge of T2. *Indicates focal area of possible active hemorrhage measuring 7 mm × 6 mm at the level of the C6-7 disc space. (b) T2-weighted axial view with arrow indicating severe spinal cord impingement corresponding with focal area of hemorrhage. Arrows in the figure represent the extent of the epidural hematoma from the mid-C2 vertebral body to T2.
Medical management: Reversal of dabigatran and avoidance of surgery
The patient’s medical management included stopping aspirin, clopidogrel, and dabigatran etexilate. After consulting with the hematology-oncology service, the patient was given idarucizumab, a direct monoclonal antibody inhibitor to dabigatran. In addition, he was started on intravenous dexamethasone 6 mg every 6 hours and gabapentin 100 mg 3 times daily.
Follow-up
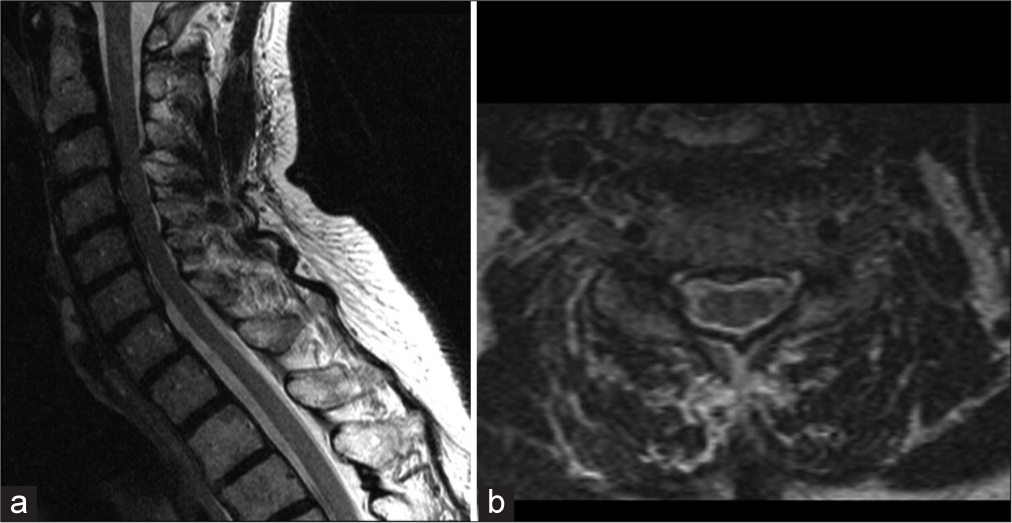
Within 2 weeks, the patient regained normal (i.e., his baseline) neurological function. The MRI obtained 1 month later (further confirmed complete resolution of the ventral epidural hematoma with no residual hemorrhage) [
DISCUSSION
NOACs
NOACs which were originally indicated for atrial fibrillation are now widely used for deep vein thrombosis and pulmonary embolism. A study of intracranial bleeding due to blunt trauma in patients taking anticoagulants showed that the mortality and surgical intervention rates were significantly lower in the group receiving NOACs than in the warfarin group.[
Dabigatran and idarucizumab
In 2015, idarucizumab was approved as the first agent for antagonizing dabigatran. It is a humanized monoclonal antibody fragment that has 350 times higher affinity to dabigatran compared to thrombin. Therefore, it antagonizes the effects of dabigatran within minutes.[
Dabigatran and spinal hematomas
This case illustrates the relatively rare presentation of an extensive and spontaneous cervical epidural hematoma in a patient taking dabigatran. The presence of aspirin and clopidogrel can potentiate this problem [
CONCLUSION
An 86-year-old male on dabigatran and antiplatelet aggregates (i.e., aspirin and clopidogrel) presented with an acute quadriparesis attributed to a spontaneous C2-T2 MR-documented ventral spinal epidural hematoma with marked cord compression. Through the administration of idarucizumab, a dabigatran antagonist, and the cessation of aspirin and clopidogrel, the patient’s neurological deficit resolved over a 2-week period, without the need for surgery.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Kim is a consultant for Medtronic and Johnson & Johnson.
References
1. Bamps S, Decramer T, Vandenbussche N, Verhamme P, Thijs V, Van Loon J. Dabigatran-associated spontaneous acute cervical epidural hematoma. World Neurosurg. 2015. 83: 257-8
2. Caputo AM, Gottfried ON, Nimjee SM, Brown CR, Michael KW, Richardson WJ. Spinal epidural hematoma following epidural steroid injection in a patient treated with dabigatran: A case report. JBJS Case Connect. 2013. 3: e64
3. Dumkow LE, Voss JR, Peters M, Jennings DL. Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. Am J Health Syst Pharm. 2012. 69: 1646-50
4. Gulati D, Dua D, Torbey MT. Hemostasis in intracranial hemorrhage. Front Neurol. 2017. 8: 80
5. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Abraham A. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in Germany-Updated series of 120 cases. Int J Stroke. 2020. 15: 609-18
6. Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012. 108: 217-24
7. Maruyama S, Hayakawa K, Kanayama S, Iwamura H, Wada D, Saito F. Idarucizumab for a traumatic head injury patient taking dabigatran. Int J Emerg Med. 2018. 11: 41
8. Mathais Q, Esnault P, Cruc M, Bernard C, Prunet B, Gaillard PE. Spontaneous cervical spinal epidural hematoma associated with dabigatran. World Neurosurg. 2018. 112: 264-6
9. Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA. Idarucizumab for dabigatran reversal. N Engl J Med. 2015. 373: 511-20
10. Ross B, Miller MA, Ditch K, Tran M. Clinical experience of life-threatening dabigatran-related bleeding at a large, tertiary care, academic medical center: A case series. J Med Toxicol. 2014. 10: 223-8
11. Truumees E, Gaudu T, Dieterichs C, Geck M, Stokes J. Epidural hematoma and intraoperative hemorrhage in a spine trauma patient on pradaxa (dabigatran). Spine (Phila Pa 1976). 2012. 37: E863-5
12. Wolfe AR, Faroqui RM, Visvikis GA, Mantello MT, Perel AB, Tewari SO. Spinal subarachnoid and subdural hematoma presenting as a Brown-Séquard-like myelopathy following minor trauma in a patient on dabigatran etexilate. Radiol Case Rep. 2017. 12: 257-60