- Department of Surgery, Division of Neurosurgery, University of Toledo, Ohio, United States.
- Department of Neurosurgery, Promedica, Toledo, Ohio, United States.
Correspondence Address:
Jordan N. Norris, Department of Surgery, Division of Neurosurgery, University of Toledo, Toledo, Ohio, United States.
DOI:10.25259/SNI_58_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jordan N. Norris1, Andrew L. Waack1, Kathryn N. Becker1, Myles Keener1, Alastair Hoyt1, Kevin Reinard2. Glioblastoma in pregnant patient with pathologic and exogenous sex hormone exposure and family history of high-grade glioma: A case report and review of the literature. 12-May-2023;14:169
How to cite this URL: Jordan N. Norris1, Andrew L. Waack1, Kathryn N. Becker1, Myles Keener1, Alastair Hoyt1, Kevin Reinard2. Glioblastoma in pregnant patient with pathologic and exogenous sex hormone exposure and family history of high-grade glioma: A case report and review of the literature. 12-May-2023;14:169. Available from: https://surgicalneurologyint.com/surgicalint-articles/12321/
Abstract
Background: Glioblastoma (GBM) incidence is higher in males, suggesting sex hormones may influence GBM tumorigenesis. Patients with GBM and altered sex hormone states could offer insight into a relationship between the two. Most GBMs arise sporadically and heritable genetic influence on GBM development is poorly understood, but reports describing familial GBM suggest genetic predispositions exist. However, no existing reports examine GBM development in context of both supraphysiologic sex hormone states and familial predisposition for GBM. We present a case of isocitrate dehydrogenase (IDH)-wild type GBM in a young pregnant female with polycystic ovary syndrome (PCOS), history of in vitro fertilization (IVF), and significant family history of GBM and further discuss how unique sex hormone states and genetics may affect GBM development or progression.
Case Description: A 35-year-old pregnant female with PCOS and recent history of IVF treatment and frozen embryo transfer presented with seizure and headache. Imaging revealed a right frontal brain mass. Molecular and histopathological analysis of the resected tumor supported a diagnosis of IDH-wild type GBM. The patient’s family medical history was significant for GBM. Current literature indicates testosterone promotes GBM cell proliferation, while estrogen and progesterone effects vary with receptor subtype and hormone concentration, respectively.
Conclusion: Sex hormones and genetics likely exert influence on GBM development and progression that may compound with concurrence. Here, we describe a unique case of GBM in a young pregnant patient with a family history of glioma and atypical sex hormone exposure due to endocrine disorder and pregnancy assisted by exogenous IVF hormone administration.
Keywords: Familial glioma, Glioblastoma, In vitro fertilization, Polycystic ovary syndrome, Pregnancy
INTRODUCTION
A sexual dimorphism exists in glioblastoma (GBM) incidence, with men accounting for 1.6 times more diagnoses than women.[
Genetic predisposition to GBM is rare and largely occurs in association with syndromes such as Li-Fraumeni and neurofibromatosis.[
CASE DESCRIPTION
A 35-year-old pregnant female with past medical history of multiple miscarriages, PCOS, and IVF treatment presented to the emergency department following a generalized tonic-clonic seizure. On presentation, the patient was mildly post-ictal and reported headache and feeling lightheaded. The patient underwent frozen embryo transfer 9 weeks before presentation following a routine hormone protocol of daily oral and transdermal estradiol (E2), progesterone injections, and leuprorelin. At presentation, the patient remained on progesterone and enoxaparin injections in support of her pregnancy. She had no personal history of neurologic dysfunction, but family history was significant for GBM in her father and an unspecified brain tumor in a paternal aunt.
Computed tomography of the head demonstrated a 4 cm mass with significant vasogenic edema and minor mass effect but no midline herniation. The patient’s obstetrician was informed and agreed to transfer to neurosurgery; a maternal fetal medicine consult approved starting the patient on levetiracetam. Subsequent noncontrast magnetic resonance imaging corroborated a 4.3 × 2.8 cm right frontal lobe mass with associated vasogenic edema, mass effect, and probable involvement of the right side of the corpus callosum without crossing the midline.
The patient underwent a right frontal craniotomy with maximal surgical debulking of the mass. She was discharged 4 days later and started Stupp protocol 36 days later following a dilation and curettage procedure for pregnancy termination. Final tumor pathology confirmed diagnosis of IDH-wild type GBM with genomic alterations including: disruption of SOX2, gain of function in estimated glomerular filtration rate (EGFR), homozygous loss of function in cyclin-dependent kinase inhibitor 2A and cyclin-dependent kinase inhibitor 2B (CDKN2B), and loss of function of phosphate and TENsin homolog deleted on chromosome 10 (PTEN).
DISCUSSION
This case provides a unique presentation of GBM in which several novel theories regarding GBM risk factors are represented. Here, we discuss the impact of sex hormones – through PCOS, pregnancy, and IVF – and heritable genetic risk on the development and growth of GBM. To the best of our knowledge, no reports of glioma in a pregnant woman following IVF for PCOS-related infertility exist in the current literature. This patient’s family history of GBM makes this case particularly distinct.
Androgens
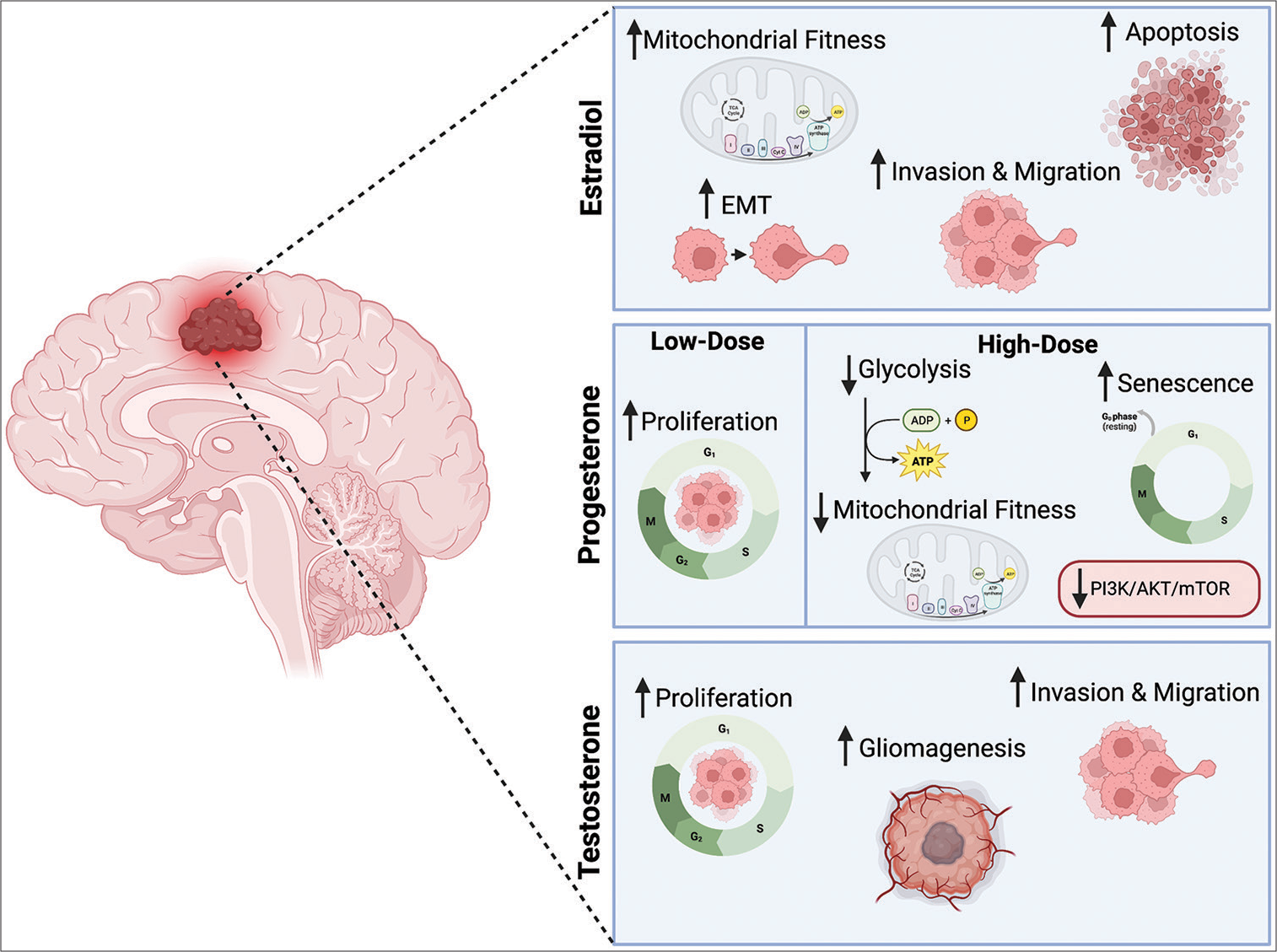
Testosterone and its metabolite dihydrotestosterone are well documented promoters of carcinogenesis and literature suggests this pro-cancer effect also applies to GBM. An initial animal study in 1970 showed that castrated rats had less induction of gliomas than non-castrated rats, implying androgen involvement in gliomagenesis.[
Figure 1:
Summary of demonstrated hormone effects on glioblastoma.
EMT: Epithelial-mesenchymal transition, PI3K: Phosphatidylinositol 3-kinase, AKT: Protein kinase B, mTOR: Mammalian target of rapamycin, ADP: Adenosine diphosphate, ATP: Adenosine triphosphate, TCA cycle: Tricarboxylic acid cycle, CytC: Cytochrome C
Estrogens
A closer look at GBM incidence by age group reveals that the characteristic sexual dimorphism is greatest at the age-range of menarche, remains constant through adulthood, and decreases following menopause.[
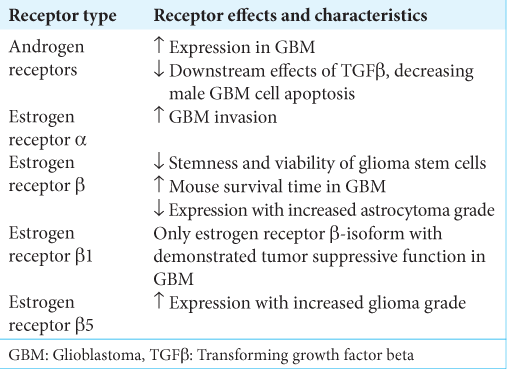
A possible explanation for the conflicting results is that the ER type modulates the downstream effects of E2. Several studies demonstrate that estrogen’s effects on GBM depend largely on which ER subtype, estrogen receptors alpha (ERα) or Estrogen receptor beta (ERβ), is targeted. Hernandez-Vega et al. found increased migration, invasion, and epithelialmesenchymal transition markers in human-derived GBM cell lines exposed to E2, suggesting that E2 may promote GBM invasion. They further demonstrated that E2 promotes GBM invasion through ERα, but not ERBERB.[
These studies suggest a protective role of ERB, but it is worth noting that ERB has five isoforms (ERB1–ERB5), each with documented distinct effects on different cancers. For example, ERB5 expression is correlated with better survival in breast cancer and non-small-cell lung cancer yet associated with poor prognosis in prostate cancer.[
Although increased androgens are a hallmark of PCOS, there may also be disruptions of E2 and ERs. Specifically, ERα knockout mouse models are associated with the development of ovarian cysts and anovulation that mimics PCOS. ERB knockouts also experiencing decreased ovulation.[
Progesterone
Evidence suggests that the effects of progesterone on GBM are concentration dependent. For example, Atif et al. found that high-dose progesterone decreased GBM growth, attenuated glycolysis, induced cellular senescence, inhibited the P13K/ Akt/mTOR pathway, and improved survival, while lower dose progesterone had no impact on tumor size.[
Pregnancy
One of the many changes that occur during gestation is a steady increase in E2 and progesterone, both of which peak in concentration during the third trimester.[
Teratogenicity of cancer therapies is a significant barrier to GBM care during pregnancy, as surgery, anesthesia, radiation, and chemotherapy can all pose at least some level of risk to the fetus. The ethical dilemmas that arise when treating a pregnant patient with GBM are especially difficult to navigate given that the average survival time after GBM diagnosis exceeds the length of gestation by only a few months, often eliminating the option to postpone cancer treatment until after delivery.[
Non-hormonal factors
While gonadal steroid hormones likely contribute to the sexual disparity in GBM incidence, other sex-specific differences in gene expression and immune function may also play a role. Sex differences in GBM incidence vary by cell type, with mesenchymal GBM cells displaying a larger sexual dimorphism than neural, proneural, or classical subsets of GBM. Greater inactivation of the tumor suppressor RB in males may be responsible for the augmented tumorigenesis and growth of mesenchymal GBM in male mouse models compared with their female counterparts.[
Heritable factors
Inheritance of GBM is uncommon and often associated with Li Fraumeni syndrome, neurofibromatosis, or Turcot syndrome.[
The genomic alterations identified in this case of GBM included disruption of SOX2, gain of function in EGFR, homozygous loss of function in CDKN2A and CDKN2B, and loss of function of PTEN. This analysis is notably limited by a lack of genetic characterization of the tumors from both the patient’s father and paternal aunt. More reports detailing familial occurrences of GBM with DNA sequencing are needed to further elucidate the contributions of nonsyndromic genetic heritability in GBM as genetic findings thus far are diverse and of limited relevance to this small subset of GBM.
CONCLUSION
This case describes a unique presentation of GBM in a young female patient with PCOS, history of IVF treatment, pregnancy, and significant family history of GBM and brain tumor. To the best of our knowledge, there are no previous reports of GBM presenting within this context. The existing literature poorly characterizes the relationship between these factors and further research is necessary to improve GBM survival and elucidate associations among GBM, genetics, biological sex differences, pathologic and supraphysiologic hormonal states, and pregnancy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Altinoz MA, Ucal Y, Yilmaz MC, Kiris İ, Ozisik O, Sezerman U. Progesterone at high doses reduces the growth of U87 and A172 glioblastoma cells: Proteomic changes regarding metabolism and immunity. Cancer Med. 2020. 9: 5767-80
2. Arruda WO, Clemente RS, Ramina R, Pedrozo AA, Pilotto RF, Pinto Júnior W. Familial glioblastoma. Arq Neuropsiquiatr. 1995. 53: 312-7
3. Atif F, Yousuf S, Espinosa-Garcia C, Sergeeva E, Stein DG. Progesterone treatment attenuates glycolytic metabolism and induces senescence in glioblastoma. Sci Rep. 2019. 9: 988
4. Atif F, Yousuf S, Stein DG. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J Steroid Biochem Mol Biol. 2015. 146: 62-73
5. Backes C, Harz C, Fischer U, Schmitt J, Ludwig N, Petersen BS. New insights into the genetics of glioblastoma multiforme by familial exome sequencing. Oncotarget. 2015. 6: 5918-31
6. Batistatou A, Stefanou D, Goussia A, Arkoumani E, Papavassiliou AG, Agnantis NJ. Estrogen receptor beta (ERbeta) is expressed in brain astrocytic tumors and declines with dedifferentiation of the neoplasm. J Cancer Res Clin Oncol. 2004. 130: 405-10
7. Bayik D, Zhou Y, Park C, Hong C, Vail D, Silver DJ. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov. 2020. 10: 1210-25
8. Bello-Alvarez C, Moral-Morales AD, González-Arenas A, Camacho-Arroyo I. Intracellular progesterone receptor and cSrc protein working together to regulate the activity of proteins involved in migration and invasion of human glioblastoma cells. Front Endocrinol (Lausanne). 2021. 12: 640298
9. Castracani CC, Longhitano L, Distefano A, Anfuso D, Kalampoka S, La Spina E. Role of 17b-estradiol on cell proliferation and mitochondrial fitness in glioblastoma cells. J Oncol. 2020. 2020: 2314693
10. González-Arenas A, Valadez-Cosmes P, Jiménez-Arellano C, López-Sánchez M, Camacho-Arroyo I. Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/ STAT6 pathway. J Steroid Biochem Mol Biol. 2014. 144: 463-70
11. Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: Reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014. 14: 3-8
12. Hernández-Vega AM, Del Moral-Morales A, Zamora-Sánchez CJ, Piña-Medina AG, González-Arenas A, Camacho-Arroyo I. Estradiol induces epithelial to mesenchymal transition of human glioblastoma cells. Cells. 2020. 9:
13. Hodgson SV, Maher ER, Hodgson S, editors. A Practical Guide to Human Cancer Genetics. Germany: Springer; 1999. 2:
14. Hopewell JW. The effects of castration on the induction of experimental gliomas in male rats. Br J Cancer. 1970. 24: 187-90
15. Kinnersley B, Mitchell JS, Gousias K, Schramm J, Idbaih A, Labussière M. Quantifying the heritability of glioma using genome-wide complex trait analysis. Sci Rep. 2015. 5: 17267
16. Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004. 23: 101-11
17. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998. 95: 15677-82
18. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009. 27: 740-5
19. Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proc Natl Acad Sci U S A. 2006. 103: 13162-7
20. Lis A, Ciesielski MJ, Barone TA, Scott BE, Fenstermaker RA, Plunkett RJ. 2-Methoxyestradiol inhibits proliferation of normal and neoplastic glial cells, and induces cell death, in vitro. Cancer Lett. 2004. 213: 57-65
21. Liu Z, Liao Y, Tang H, Chen G. The expression of estrogen receptors b2, 5 identifies and is associated with prognosis in non-small cell lung cancer. Endocrine. 2013. 44: 517-24
22. McKinley BP, Michalek AM, Fenstermaker RA, Plunkett RJ. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg. 2000. 93: 932-9
23. Mukherjee S, Stroberg E, Wang F, Morales L, Shan Y, Rao A. SMARCB1 gene mutation predisposes to earlier development of glioblastoma: A case report of familial GBM. J Neuropathol Exp Neurol. 2020. 79: 562-5
24. Nomura M, Yamagishi S, Harada S, Yamashima T, Yamashita J, Yamamoto H. Placenta growth factor (PlGF) mRNA expression in brain tumors. J Neurooncol. 1998. 40: 123-30
25. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021. 23: 1-iii105
26. Ostrom QT, Coleman W, Huang W, Rubin JB, Lathia JD, Berens ME. Sex-specific gene and pathway modeling of inherited glioma risk. Neuro Oncol. 2019. 21: 71-82
27. Pallud J, Mandonnet E, Deroulers C, Fontaine D, Badoual M, Capelle L. Pregnancy increases the growth rates of World Health Organization grade II gliomas. Ann Neurol. 2010. 67: 398-404
28. Peeters S, Pagès M, Gauchotte G, Miquel C, Cartalat-Carel S, Guillamo JS. Interactions between glioma and pregnancy: Insight from a 52-case multicenter series. J Neurosurg. 2018. 128: 3-13
29. Piña-Medina AG, Hansberg-Pastor V, González-Arenas A, Cerbón M, Camacho-Arroyo I. Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids. 2016. 105: 19-25
30. Reed MR, Lyle AG, De Loose A, Maddukuri L, Learned K, Beale HC. A functional precision medicine pipeline combines comparative transcriptomics and tumor organoid modeling to identify bespoke treatment strategies for glioblastoma. Cells. 2021. 10:
31. Rodríguez-Lozano DC, Piña-Medina AG, Hansberg-Pastor V, Bello-Alvarez C, Camacho-Arroyo I. Testosterone promotes glioblastoma cell proliferation, migration, and invasion through androgen receptor activation. Front Endocrinol (Lausanne). 2019. 10: 16
32. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Gro. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004. 81: 19-25
33. Sander C, Reuschel V, Eisenlöffel C, Nestler U, Meixensberger J. Familial glioblastoma clustering in adult patients: A case report of two non-twin siblings and review of the literature. Int Med Case Rep J. 2019. 12: 205-11
34. Sareddy GR, Li X, Liu J, Viswanadhapalli S, Garcia L, Gruslova A. Selective estrogen receptor b agonist LY500307 as a novel therapeutic agent for glioblastoma. Sci Rep. 2016. 6: 24185
35. Sareddy GR, Pratap UP, Venkata PP, Zhou M, Alejo S, Viswanadhapalli S. Activation of estrogen receptor beta signaling reduces stemness of glioma stem cells. Stem Cells. 2021. 39: 536-50
36. Schiffgens S, Wilkens L, Brandes AA, Meier T, Franceschi E, Ermani M. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget. 2016. 7: 55169-80
37. Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE. Targeted disruption of the estrogen receptor-alpha gene in female mice: Characterization of ovarian responses and phenotype in the adult. Endocrinology. 1999. 140: 2733-44
38. Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008. 14: 5228-35
39. Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005. 84: 701-10
40. Sun T, Warrington NM, Luo J, Brooks MD, Dahiya S, Snyder SC. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest. 2014. 124: 4123-33
41. Turbyville TJ, Gürsel DB, Tuskan RG, Walrath JC, Lipschultz CA, Lockett SJ. Schweinfurthin a selectively inhibits proliferation and Rho signaling in glioma and neurofibromatosis Type 1 tumor cells in a NF1-GRD-dependent manner. Mol Cancer Ther. 2010. 9: 1234-43
42. Ugonabo I, Bassily N, Beier A, Yeung JT, Hitchcock L, De Mattia F. Familial glioblastoma: A case report of glioblastoma in two brothers and review of literature. Surg Neurol Int. 2011. 2: 153
43. Witthayanuwat S, Pesee M, Supaadirek C, Supakalin N, Thamronganantasakul K, Krusun S. Survival analysis of glioblastoma multiforme. Asian Pac J Cancer Prev. 2018. 19: 2613-7
44. Yang W, Warrington NM, Taylor SJ, Whitmire P, Carrasco E, Singleton KW. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. 2019. 11:
45. Yu X, Jiang Y, Wei W, Cong P, Ding Y, Xiang L. Androgen receptor signaling regulates growth of glioblastoma multiforme in men. Tumour Biol. 2015. 36: 967-72
46. Zhou M, Sareddy GR, Li M, Liu J, Luo Y, Venkata PP. Estrogen receptor beta enhances chemotherapy response of GBM cells by down regulating DNA damage response pathways. Sci Rep. 2019. 9: 6124