- Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez, Ciudad de México, Mexico.
- Department of Neuro-otology, National Institute of Neurology and Neurosurgery, Manuel Velasco Suárez, Ciudad de México, Mexico.
Correspondence Address:
Oscar Rubén Contreras-Vázquez, Department of Neurosurgery, National Institute of Neurology and Neurosurgery, Ciudad de México, Mexico.
DOI:10.25259/SNI_518_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rogelio Revuelta-Gutiérrez1, Fernando Piñon-Jiménez1, Oscar Rubén Contreras-Vázquez1, Lourdes Olivia Vales-Hidalgo2, Jaime Jesús Martinez-Anda1. Glossopharyngeal and vagoglossopharyngeal neuralgia: Long-term surgical outcomes in a single institution. 28-Jul-2023;14:267
How to cite this URL: Rogelio Revuelta-Gutiérrez1, Fernando Piñon-Jiménez1, Oscar Rubén Contreras-Vázquez1, Lourdes Olivia Vales-Hidalgo2, Jaime Jesús Martinez-Anda1. Glossopharyngeal and vagoglossopharyngeal neuralgia: Long-term surgical outcomes in a single institution. 28-Jul-2023;14:267. Available from: https://surgicalneurologyint.com/surgicalint-articles/12463/
Abstract
Background: Glossopharyngeal neuralgia (GPN) and vagoglossopharyngeal neuralgia (VGPN) are infrequent syndromes that can have great negative impact on a patient’s quality of life. The objective of this study is to describe the characteristics and long-term results of patients with GPN-VGPN who are treated surgically with microvascular decompression (MVD) in one institution.
Methods: This is a retrospective series of 20 patients with the diagnosis of GPN-VGPN who underwent MVD. Demographic characteristics, surgical results, complications, and long-term follow-up were analyzed.
Results: The mean age of symptom onset was 51.25 years and the majority of patients were women (60%). The posterior inferior cerebellar artery was the main offending vessel (75%). The immediate MVD success rate was 100%, but during follow-up, two patients (10%) were diagnosed with VGPN and both cases presented pain recurrence. The mean follow-up was 120.4 (25–333) months. VGPN (P = 0.005) and a ≥5 day hospital stay (P = 0.032) were associated with unsuccessful outcomes. Two complications were documented, which resolved without sequelae. There was no surgical mortality.
Conclusion: MVD is an effective and safe treatment for long-term pain relief of GPN-VGPN. VGPN and a prolonged hospital stay were associated with poor outcomes. More studies are required to confirm these findings.
Keywords: Glossopharyngeal neuralgia, Microvascular decompression, Vagoglossopharyngeal neuralgia
INTRODUCTION
Glossopharyngeal neuralgia (GPN), formerly known as vagoglossopharyngeal neuralgia (VGPN),[
The pathophysiology of classic GPN has been known for several decades, the etiology of which is usually vascular compression in the root entry zone (REZ) in 95% [
Figure 1:
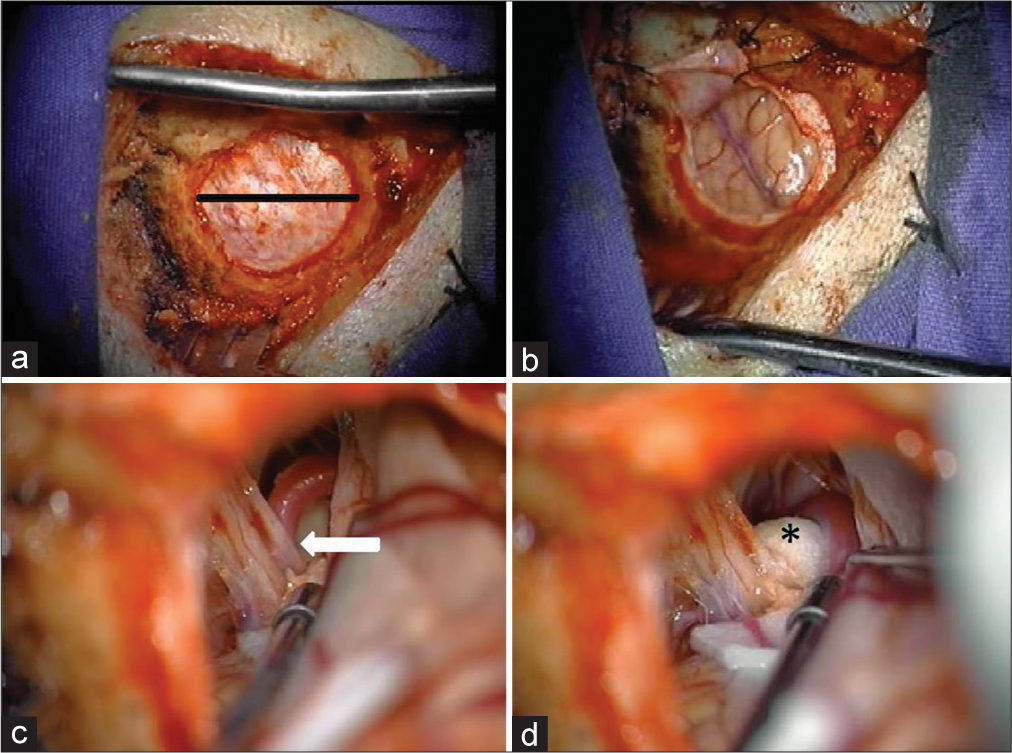
Microvascular decompression through a keyhole craniectomy in a microasterional approach. (a) Microasterional craniotomy performed with a diameter of 2.5 cm exposes the dura with autostatic retractors (black line). (b) Cerebellar hemisphere is exposed after dural opening. (c) The root entry zone and nerve pathway are identified through blunt dissection with a suction and bipolar device, where neurovascular conflict with posterior inferior cerebellar artery is observed (white arrow). (d) Teflon is placed between the nerve pathway and the offending vessel to maintain vessel retraction and avoid vascular contact (*).
Microvascular decompression (MVD) is the main surgical technique performed. It has shown good results, with success rates of around 90%. However, given the low incidence of GPNVGPN, there are currently few studies which identify, describe, and analyze prognostic factors in a long-term cohort.[
Our case series aims to describe the characteristics and long-term outcomes of patients diagnosed with GPN-VGPN who were surgically treated with MVD in a single institution.
MATERIALS AND METHODS
This retrospective study was approved by the Institution’s Ethics and Research Committees. Only patients with an established diagnosis of classical GPN who met the current International Headache Society 2018 criteria[
Data recollection and follow-up
The medical record information was obtained by an investigator other than the surgeon Rogelio RevueltaGutiérrez to avoid bias. Patient data including gender, age of symptom onset, accompanying symptoms, prior medical treatments, time from symptom onset to surgery, operative findings, complications, immediate clinical outcome, and long-term follow-up were analyzed. The follow-up was carried out by obtaining information from the last clinical note in their medical records and/or by telephone calls.
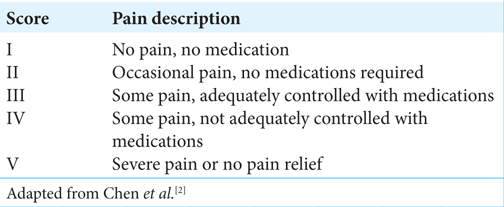
The Barrow Neurological Institute Pain Intensity Score (BNIPIS) was used to classify pain intensity [
Statistical analyses
A descriptive analysis of the variables obtained from the sample of patients with GPN and VGPN was performed using the SPSS, IBM software, version 26. The results of the nominal, ordinal, and quantitative categorical variables were described using measures of central tendency. After the descriptive statistical analysis was completed, an inferential statistical test was carried out to identify risk factors associated with a poor surgical outcome.
Surgical technique
Under general anesthesia, the patients were placed in the Park Bench position. The upper part of the shoulder was retracted and the head was rotated 60° to the opposite side of the incision site, with a slight lateral cervical inclination of 10° toward the floor to form an optimal surgical corridor. A 5 cm retrosigmoid incision centered over the asterion was performed, and a keyhole microasterional craniectomy (2.5–3 cm) was made, exposing the junction of the transverse and sigmoid sinuses [
RESULTS
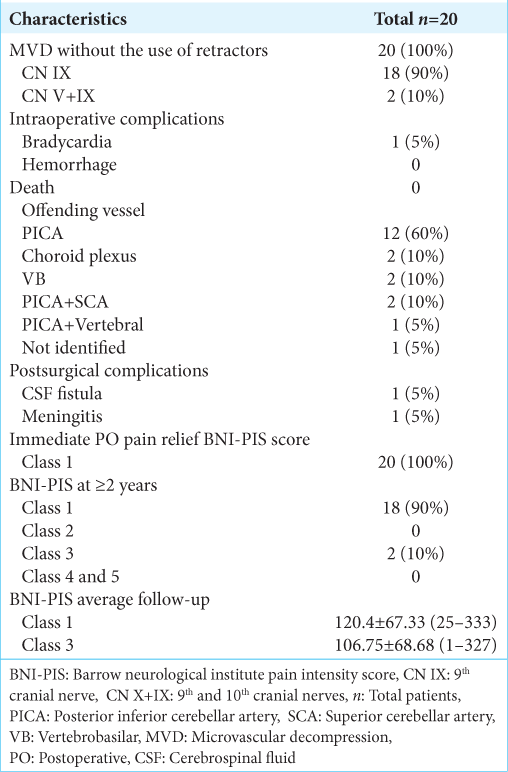
During the period from January 1, 1994, to February 1, 2023, a total of 20 patients were diagnosed with GPN at the INNN neurosurgery department and were all treated using microasterional MVD approach without the use of retractors [
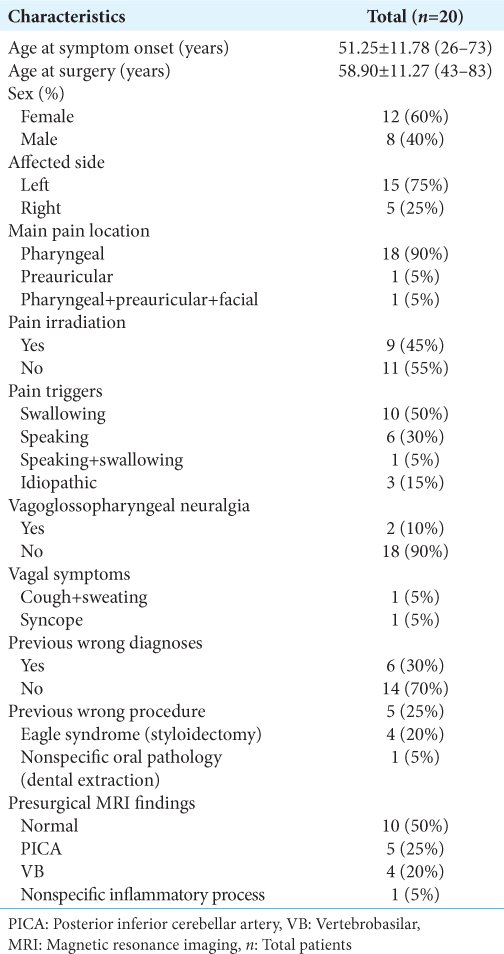
The average age of symptom onset was 51.25 ± 11.7 (26–73) years. Surgery was performed after a median of 5 years after diagnosis. The mean age at surgery was 58.9 ± 11.27 (43–83) years.
In 15 patients (75%), the affected side was the left, while the right side was affected in 5 patients (25%). The primary location of pain was pharyngeal in 18 patients (90%), followed by preauricular in 1 patient (5%), and pain in the pharyngeal, preauricular, and facial regions in 1 patient (5%).
Swallowing was the main trigger (ten patients – 50%), followed by speaking (six patients - 30%), with only one patient presenting both. No trigger was identified in 3 patients (15%).
Presurgical erroneous diagnoses were made in 6 patients (30%) and an erroneous procedure was performed in 5 patients (25%) without pain improvement in extrainstitutional health care centers. Four of these patients were diagnosed with Eagle Syndrome and underwent styloidectomy. One patient was diagnosed with unspecified oral pathology, treated with multiple dental extractions which caused him chewing alterations. One patient was diagnosed with TN and had no surgical procedure performed; however, he was treated pharmacologically for more than 5 years under the diagnosis of atypical TN that affected V1, predominantly in the preauricular region, and radiated to V2 and pharynx, his pain was triggered by speaking and swallowing. During the presurgical protocol, the clinical diagnosis of GPN was made based on the type of symptoms and radiological findings (vertebrobasilar [VB] dolichoectasia).
Two patients (10%) were diagnosed with VGPN based on the associated symptoms during their pain episodes. One of them presented sweating and coughing and the other presented syncope.
All the patients were treated pharmacologically for at least 6 months, without obtaining a favorable response. The most often used pharmacological regimen was CBZ in monotherapy (65%), followed by dual therapy with pregabalin and CBZ (15%), and the remaining 20% with a combination of more than three drugs. The resistance and/or intolerance to pharmacological treatment was considered a criteria for surgical treatment.
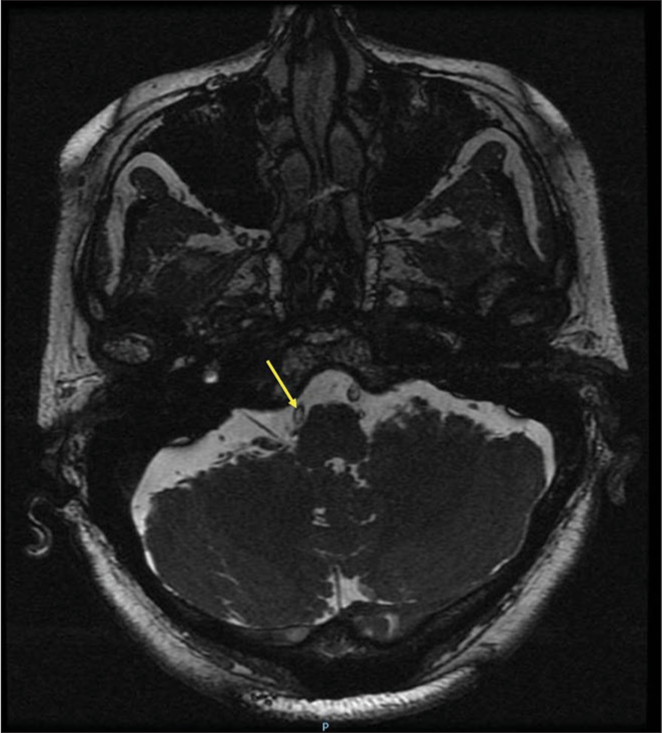
Magnetic resonance imaging (MRI) was performed in all patients, with the purpose of ruling out other etiologies. Half of the patients presented a normal MRI, with the presence of vascular conflict in the REZ of the glossopharyngeal nerve present in less than half of the patients [
Surgical outcomes
All patients underwent MVD for the 1st time, using a microasterional approach without the use of CR [
The main offending vessel identified during surgery was the PICA in 15 patients (75%). It was found to be independent in 12 patients (60%) and associated with the superior cerebellar artery in 2 patients (10%) and with the vertebral artery (VA) in 1 patient (5%). The choroid plexus was responsible for the compression in 2 patients (10%) and the VB complex in another 2 patients (10%). No offender vessel was identified in one patient (5%).
Only one patient had intraoperative bradycardia, with no further complications. No other intraoperative complications were presented.
In the immediate postoperative (PO) period, all patients presented complete pain relief. Two patients (10%) presented postoperative complications. One presented a CSF fistula which was treated with lumbar punctures and acetazolamide, which was remitted completely without requiring surgical reintervention. Another patient presented meningitis which was treated with intravenous antibiotics, with full remission and negative cultures 2 weeks after, and was discharged without any further complications.
Long-term follow-up
On average, patient follow-up was of 120.40 ± 67.33 (range, 25–333) months. A total of 18 patients (90%) reported complete pain relief during the follow-up visits (BNIPIS 1). Only 2 patients (10%) referred pain recurrence in the outpatient clinic at the 1 month and 20 months follow-up. In both cases, monotherapy was started with minimal dosage of CBZ, achieving pain control (BNIPIS 3) without reintervention criteria. Two factors were associated with unfavorable outcomes (BNIPIS 3), vagal affection (P = 0.005), and a hospital stay of 5 or more days (P = 0.032).
DISCUSSION
This study demonstrated that MVD is an effective treatment for long-term pain relief with an effectiveness rate of 90%, minor complications, and no mortality. In our series, the most common offending vessel was the PICA (60%), either alone or in combination with other vessels. Inoue et al.[
During follow-up, we found that the two patients that referred pain recurrence were diagnosed with VGPN, which showed statistical significance by Fisher’s exact test (P = 0.005). Although both patients were female, no statistical association was found between sex and surgical outcome. Our experience in the INNN, as exposed in other authors’ reports, shows that the term VGPN could be used when GPN is associated with autonomic symptoms due to vagal dysfunction.[
The pathophysiology of VGPN, as explained by Elias et al.[
Palanisamy et al.[
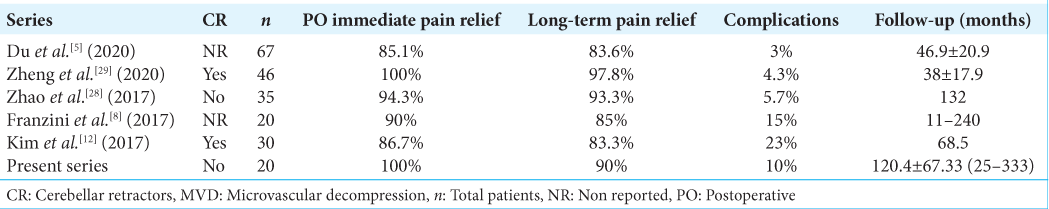
At present, there are few reports with long-term follow-up in patients with GPN and VGPN who underwent MVD, which are shown in
We present all the series of cases where MVD was performed, and a long-term follow-up was reported [
There is still much debate about which treatment is superior. Some authors advocate rhizotomy as the first-line surgical treatment due to its slightly higher rates of long-term pain relief; however, as it is a more invasive procedure, high rates of serious and permanent PO complications such as dysphagia and vocal cord paralysis have been described.[
On the other hand, Shimizu et al.,[
Pommier et al.[
Finally, although MVD appears to be a promising treatment option, further studies are required to identify the particular aspects of these syndromes in greater detail and thus be able to establish different surgical profiles that would allow us to offer better clinical outcomes to our patients.
CONCLUSION
MVD has proven to be a safe and effective treatment option for GPN and VGPN, as it entails fewer and minor complications and overall lasting pain remission, despite the fact that surgeon experience is an influential factor on surgical outcomes. The present study is particularly transcendent due to its longer-term follow-up, as well as its unique surgical technique, in which CR and intraoperative neurophysiological monitoring are not used. However, the dispersion of follow-up length, together with the sample size, limits the value of the statistical results. It should be noted that the preoperative approach is essential for an ideal surgical outcome, so particular attention must be paid to atypical facial pain syndromes as to avoid iatrogenic causes. In addition, performing a good surgical technique, with a detailed inspection of the clinically associated nerve path, offers the best scenario for the remission of the patient’s pain.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Chawla JC, Falconer MA. Glossopharyngeal and vagal neuralgia. Br Med J. 1967. 3: 529-31
2. Chen HI, Lee JY. The measurement of pain in patients with trigeminal neuralgia. Clin Neurosurg. 2010. 57: 129-33
3. Chen J, Sindou M. Vago-glossopharyngeal neuralgia: A literature review of neurosurgical experience. Acta Neurochir (Wien). 2015. 157: 311-21 discussion 321
4. Dalessio DJ. Diagnosis and treatment of cranial neuralgias. Med Clin North Am. 1991. 75: 605-15
5. Du T, Ni B, Shu W, Hu Y, Zhu H, Li Y. Neurosurgical choice for glossopharyngeal neuralgia: A benefit-harm assessment of long-term quality of life. Neurosurgery. 2020. 88: 131-9
6. Elias J, Kuniyoshi R, Carloni WV, Borges MR, Peixoto CA, Pimentel D. Glossopharyngeal neuralgia associated with cardiac syncope. Arq Bras Cardiol. 2002. 78: 510-9
7. Fraioli B, Esposito V, Ferrante L, Trubiani L, Lunardi P. Microsurgical treatment of glossopharyngeal neuralgia: Case reports. Neurosurgery. 1989. 25: 630-2
8. Franzini A, Messina G, Franzini A, Marchetti M, Ferroli P, Fariselli L. Treatments of glossopharyngeal neuralgia: Towards standard procedures. Neurol Sci. 2017. 38: 51-5
9. , editors. Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders. Cephalalgia. 2018. 38: 1-211
10. Inoue K, Matsushima T, Ohara S, Masuoka J, Abe T. Study of the anatomical features of the offending arteries involved in glossopharyngeal neuralgia. Oper Neurosurg (Hagerstown). 2020. 19: E259-68
11. Iwai Y, Ishibashi K, Yamanaka K. Gamma knife radiosurgery for concurrent trigeminal neuralgia and glossopharyngeal neuralgia. Cureus. 2021. 13: e20717
12. Kim MK, Park JS, Ahn YH. Microvascular decompression for glossopharyngeal neuralgia: Clinical analyses of 30 cases. J Korean Neurosurg Soc. 2017. 60: 738-48
13. Krasoudakis A, Anyfantakis D, Hadjipetrou A, Kastanakis M, Symvoulakis EK, Marathianos S. Glossopharyngeal neuralgia associated with cardiac syncope: Two case reports and literature review. Int J Surg Case Rep. 2015. 12: 4-6
14. Ma Y, Li YF, Wang QC, Wang B, Huang HT. Neurosurgical treatment of glossopharyngeal neuralgia: Analysis of 103 cases. J Neurosurg. 2016. 124: 1088-92
15. Palanisamy D, Kyosuke M, Yasuhiro Y, Tsukasa K, Kato Y. Management of recurrent glossopharyngeal neuralgia following microvascular decompression surgery. World Neurosurg. 2018. 117: 339-43
16. Pommier B, Touzet G, Lucas C, Vermandel M, Blond S, Reyns N. Glossopharyngeal neuralgia treated by Gamma Knife radiosurgery: Safety and efficacy through long-term follow-up. J Neurosurg. 2017. 128: 1372-9
17. Revuelta-Gutiérrez R, Morales-Martínez AH, Mejías-Soto C, Martínez-Anda JJ, Ortega-Porcayo LA. Microvascular decompression for glossopharyngeal neuralgia through a microasterional approach: A case series. Surg Neurol Int. 2016. 7: 51
18. Rey-Dios R, Cohen-Gadol AA. Current neurosurgical management of glossopharyngeal neuralgia and technical nuances for microvascular decompression surgery. Neurosurg Focus. 2013. 34: E8
19. Riley HA, Greenan WJ, Wortis H. Glossopharyngeal neuralgia initiating or associated with cardiac arrest. Trans Am Neurol Assoc. 1942. 68: 28-9
20. Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004. 22: 185-206
21. Rui Y, Ji W, Chuncheng Q, Shengcheng W. Efficacy comparison of microvascular decompression and rhizotomy in the treatment of glossopharyngeal neuralgia: A retrospective analysis of 37 cases. Turk Neurosurg. 2019. 29: 493-6
22. Rushton JG, Stevens JC, Miller RH. Glossopharyngeal (vagoglossopharyngeal) neuralgia: A study of 217 cases. Arch Neurol. 1981. 38: 201-5
23. Shimizu K, Matsumoto M, Wada A, Sugiyama T, Tanioka D, Okumura H. Supine no-retractor method in microvascular decompression for hemifacial spasm: Results of 100 consecutive operations. J Neurol Surg B Skull Base. 2015. 76: 202-7
24. Taşci İ, Beydilli İ, Demir CF, Balgetir F, Gönen M, Bakir M. A case of syncopal convulsions triggered by glossopharyngeal neuralgia. Agri. 2021. 33: 197-9
25. Thomson JL. Glossopharyngeal neuralgia accompanied by unconsciousness. J Neurosurg. 1954. 11: 511-4
26. Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain. 2014. 155: 654-62
27. Wilhour D, Nahas SJ. The neuralgias. Curr Neurol Neurosci Rep. 2018. 18: 69
28. Zhao H, Zhang X, Zhu J, Tang YD, Li ST. Microvascular decompression for glossopharyngeal neuralgia: Long-term follow-up. World Neurosurg. 2017. 102: 151-6
29. Zheng X, Wei XY, Zhu J, Yuan Y, Ying TT, Li ST. Microvascular decompression alone without rhizotomy is an effective way of treating glossopharyngeal neuralgia: Clinical analysis of 46 cases. Stereotact Funct Neurosurg. 2020. 98: 129-35